- Home
- KyooBe Tech GmbH
- eFit
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
eFit
Product Description
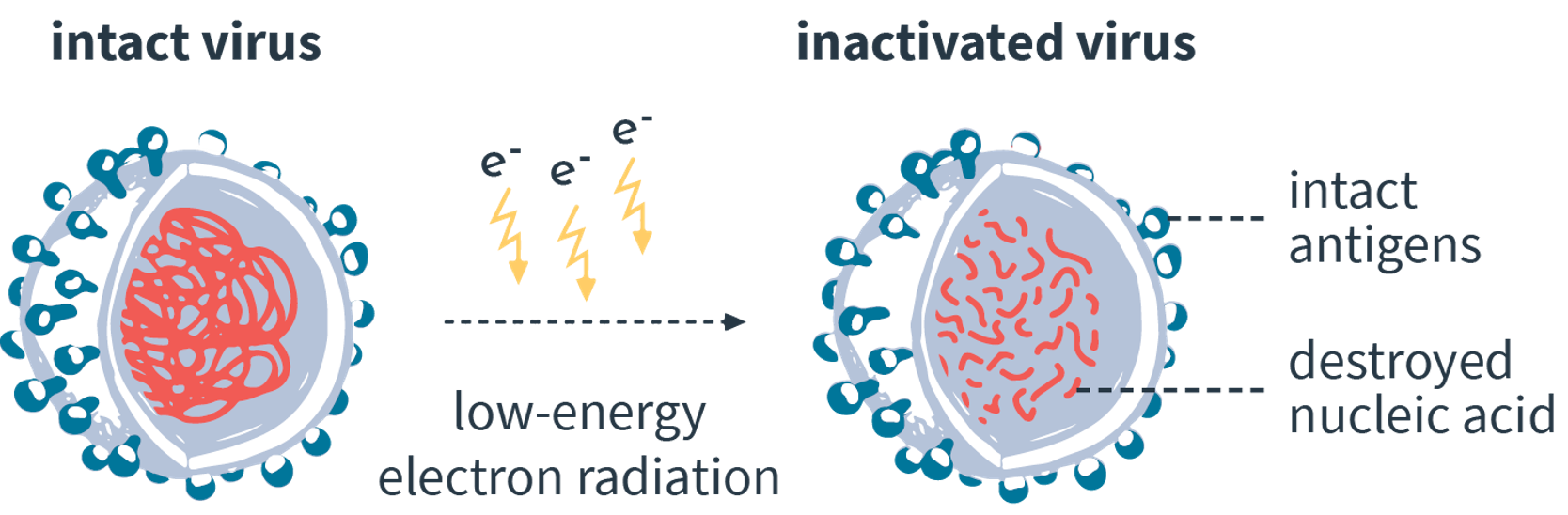
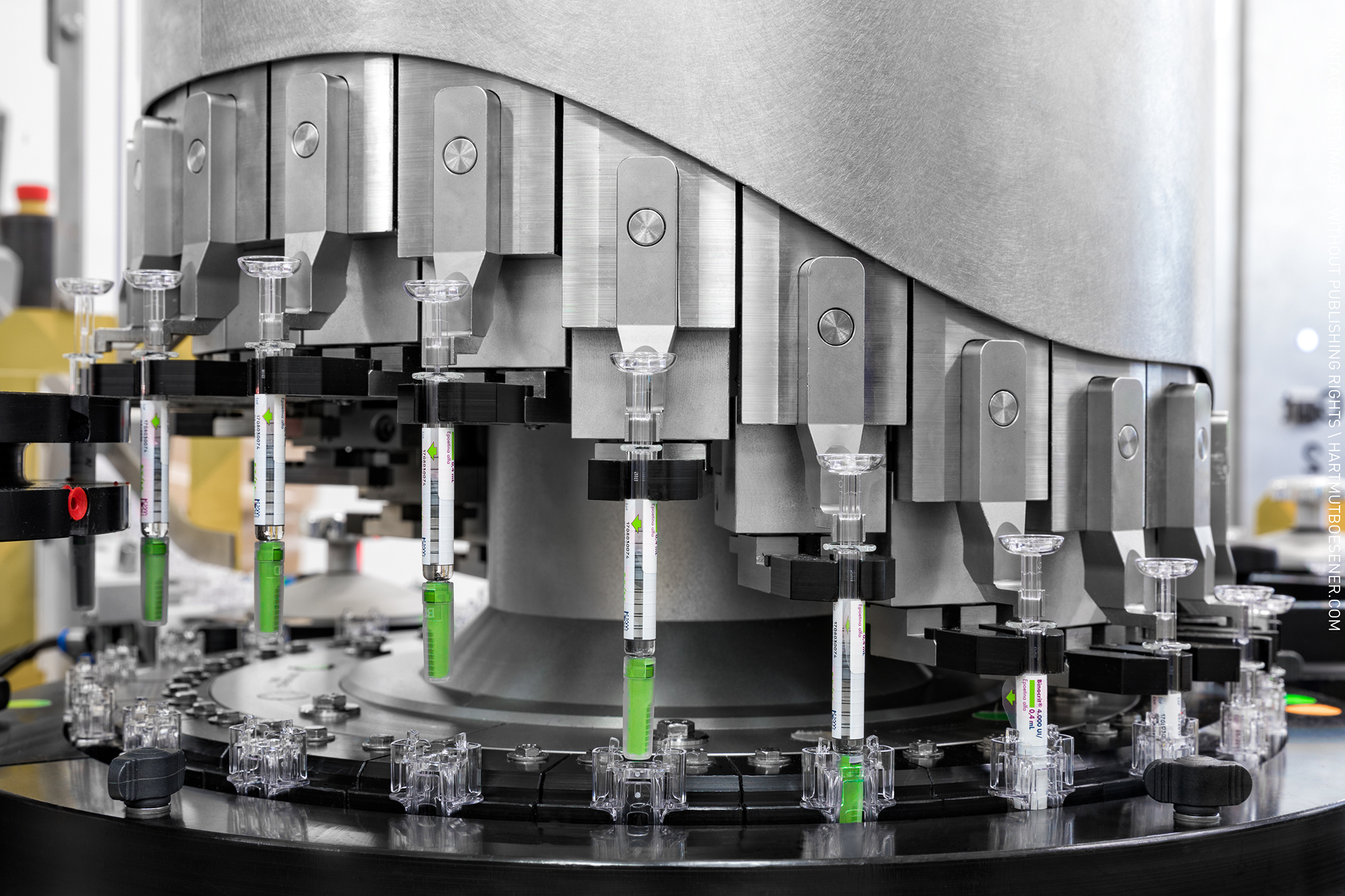
eFIT is a device for the inactivation of pathogens & cells.
By low energy electron irradiation (LEEI) the genetic material of the pathogens & cells is reliably destroyed. The eFIT platform is suitable for both research (R&D) and small-scale manufacturing companies.
- Vaccine production
- Virus depletion in process media and blood-derived components incl. sera
KyooBe Tech GmbH

-
DE
-
2022On CPHI since
-
25 - 49Employees
Company types
Biopharmaceutical company
Engineering
Primary activities
Biopharmaceutical
Laboratory, Analytical, Biotechnology Equipment
Technology
Departments
General Management
Marketing/Communications/PR
-
Gordon EisenachMarketing & Business Development ManagerLeinfelden-Echterdingen, Baden-Württemberg, Germany
Other
-
Andrea TraubeGermany
Categories
Specifications
- DetailsTechnical Specs:
- Footprint: 1400 mm x 850 mm x 2120 mm
- Weight: 1250 kg
- Power supply: 3x 400V - 16A and 1x 230V -10A
- Energy consumption: 1 kWh / h
- Batch size: 10 ml to 1 L
- Production speed: 0.33 L/h
- Radiation dosis: 1 - 60 kGy
- Depletion level: > 6 log levels reduction
- Viscosity range: 1 - 4,5 mPa*s
- Temperature range: 5-30 °C
- Selling PointsGreen Product; Immune Health; Sustainability; Relevant irradiation targets:
- Bacteria (gram+/-)
- Bacterial spores
- Viruses (enveloped, non-enveloped, DNA, RNA)
- Immunologically relevant cells
- Parasites
- Find more details here: https://kyoobe.tech/ressources/
- ModelI22
- Supplied fromGermany
- Measured Inkilogram; kilojoule; litre; metre
KyooBe Tech GmbH

-
DE
-
2022On CPHI since
-
25 - 49Employees
Company types
Biopharmaceutical company
Engineering
Primary activities
Biopharmaceutical
Laboratory, Analytical, Biotechnology Equipment
Technology
Departments
General Management
Marketing/Communications/PR
-
Gordon EisenachMarketing & Business Development ManagerLeinfelden-Echterdingen, Baden-Württemberg, Germany
Other
-
Andrea TraubeGermany
More Products from KyooBe Tech GmbH (1)
-
Product INACTIVATE - I22
INACTIVATE / I22 is a device for the inactivation of pathogens & cells of any kind. By low energy electron irradiation (LEEI) the genetic material of the pathogens & cells is reliably destroyed. The I22 device is suitable for research purposes and small industrial batches. Example use-cases are: Vaccine ...
KyooBe Tech GmbH resources (1)
-
Technical Data eFit - Product Detail Page
eFIT is a device for the inactivation of pathogens and cells in a liquid solution. By low energy electron irradiation (LEEI) the genetic material is reliably destroyed. The eFIT platform is suitable for both research (R&D) and small-scale manufacturing processes.Vaccine productionVirus depletion in process media and blood-derived components incl. sera
Frequently Viewed Together
-
Product A spray for vaccine administration through the nasal route
With our extensive experience in intranasal delivery, we offer reliable, easy to use and safe nasal vaccine solutions*. The concept device is constituted with a nozzle, a dose divider, and a luer lock syringe. It contains two-doses, allowing vaccine delivery for each nostril.
-
Product ULC Series 328
FARRAR® pioneered forced air convection cooling to answer the challenge of preserving and storing biological and human tissue donor samples and materials. Purpose-built for life science applications, the Ultra-Low Chamber ULC-190, ULC-259, and ULC-328 are the only -20°C to-80°C, forced air, ultra-low-t...
-
Product Adjuvant Systems for Vaccines
We enable next-generation vaccine development through a diverse portfolio of world-leading vaccine adjuvants, immunomodulators and adjuvant formulations.
We passionately develop new adjuvant technologies and partner to develop the vaccines of tomorrow.
Our extensive expertise in immunology and fo...
-
Product CordenPharma BotaniChol® Plant-Based Cholesterol
Chemists at CordenPharma and the Otto-von-Guericke-University of Magdeburg, Germany developed a scalable synthesis of pharma-grade cholesterol based on botanical sources. This offers a large-scale production of highly needed cholesterol, which is used as part of the lipid cocktail ...
-
Product Process Plant Master Plant
The IKA Master Plant homogenizing and emulsifying plant is a universal mixing plant developed for the production of emulsions and suspensions in the pharmaceuticals industry in particular, but also in the food, beverages, cosmetics and chemical industries. The plant is GMP-compliant and guarantees ...
-
Product Recombinant COVID-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation (...
The world's first inhaled COVID-19 vaccine Convidecia Air® has been approved as a booster dose in China, Morocco, and Indonesia.
Induces humoral, cellular, and mucosal immunity
Needle-free vaccination

-
Product Packaging, Quality Control, Storage, Cold Chain
Within the contract development and manufacturing, IDT Biologika GmbH provides a wide range of other pharmaceutical services for our clients products, which include- Labeling & Packaging of vials, prefilled syringes, autoinjectors - Serializiation (Track & Trace) - Quality Systems - Cold Chain up to - 80- ...
-
Product SEELCAP®
Our products comply with tamper-evident requirements in the pharmaceutical industry and offer increased protection against tampering by means of security perforations.Proof of authenticity and provision of security (tamper-evident)Suitability for many different packaging materials and surfacesAn integrated...
-
Product HIP-Vax
The demand for viral vector-based products is growing rapidly. However, there are still manufacturing challenges which hamper the development of these promising new therapies. A major problem is low product yields. For many medical applications, a certain minimal dose of product is needed to have a clinica...
-
Product Pharmatec GmbH Vaccine Manufacturing Line
NEW/NEVER USEDIMMEDIATELY AVAILABLE FROM A CANCELLED PROJECT Complete Pilot Size Vaccine Development Manufacturing Lines Manufacturer: Syntegon Pharmatec GmbH/Germany - 2021
- Pressure Equipment Directive (PED) 2014/68/EU- European GMP (Good Manufacturing Practice) Directives- Vessels pass...
-
Product Technology Transfer
Maximize the production capabilities through a de-risking process with our comprehensive technology transfer services for engineering batches, process performance qualification (PPQ) batches, and commercial batches of vaccine antigens, including viral vectors, protein subunits, and virus-like par...
-
Product Vaccine Characterization and Bioanalytical Support
Our vaccines development experts provide a suite of services supporting the analysis and quality control of process and batch release samples and stability studies. We have support the development of a range of vaccines including mRNA, protein, glycoprotein, DNA, carbohydrate, lipopolysaccharide, lipi...
-
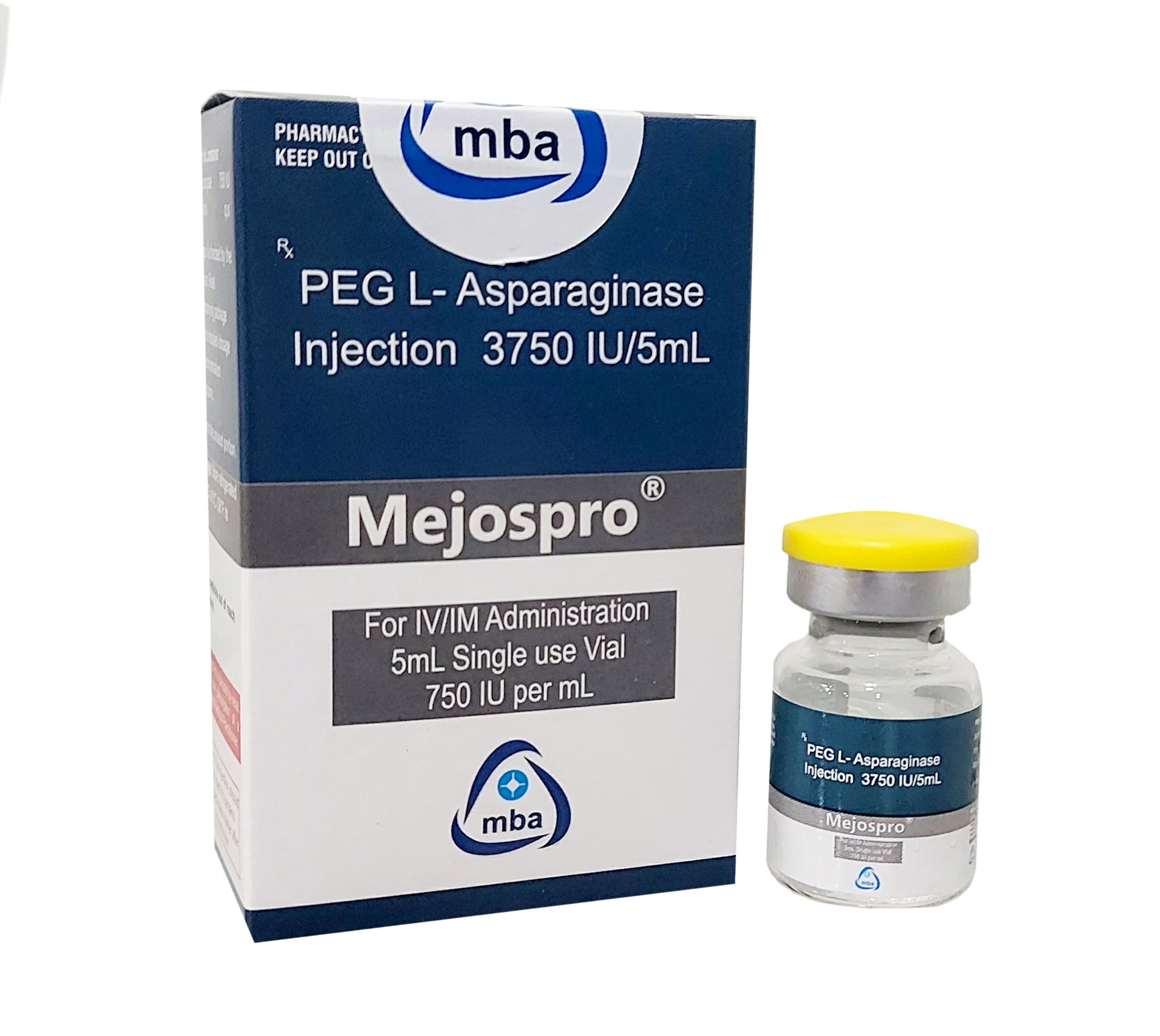
Product MEJOSPRO -Peg L Asparaginase Injection 3750IU/5ML
PEG L-Asparaginase 3750 IU.- Mejospro is used in the treatment of acute lymphoblastic leukemia.
Category: Antineoplastic
This drug is used for :
Peg L Asparaginase is used to treat acute lymphocytic leukemia (ALL).Non-Hodgkin’s lymphoma.Used in some patients...
-
Product 23-valent Pneumococcal Polysaccharide Vaccine
To prevent invasive diseases caused by 23 serotypes of Streptococcus pneumoniae for use in individuals aged ≥ 2 years who are at increased risk of pneumococcal diseases
-
Product Nested container filling and stoppering solution Ultra-N serious
Capable for Nested Syringes, Cartridges and Vials Processing
Fully Automatic De-bagging, De-lidding, Filling and Stoppering, Vials De-nesting
Output Up to 15000 pcs/h
Available for Mechanical Stoppering , Vacuum Filling and Stoppering

-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product Barycela
"World’s 1st Antibiotic-free varicella vaccine" • WHO PQ vaccine • Improved stability with automatic and aseptic process • Over the past 28 years, + 28 M doses provided • Exported more than 30 countires
• Generic name: Varicella Vaccine • Indication: For prophylaxis against varicella • Pr...
-
Product Viral Safety Testing
SGS’s global center of excellence for cell bank characterization & virus testing is located in the United Kingdom and provides services with ultimate reliability, highest GLP/cGMP quality & scientific expertise.
For any of your biologics, we help you comply with the global regula...
-
Product Clinical & Medical Monitoring
1. Clinical monitoring refers to the systematic process of assessing a patient's health status during the course of treatment or care. This involves tracking various clinical parameters, observing the effectiveness of interventions, and identifying any changes in the patient's condition.2. Medical monitori...
-
Product Lipid nanoparticles
Lipid nanoparticles (LNPs) enable the delivery of a variety of molecules, including nucleic acids such as mRNA, to cells and are therefore an essential tool in gene therapy. To unleash the potential of vaccines, protein and gene therapies, drug developers need the support of a trusted and experienced pro...
-
Product Vaccine Adjuvant: Alhydrogel™
• Alhydrogel is a range of aluminium hydroxide gel products which have been specifically developed for use as an adjuvant in human and veterinary vaccines • The gel is a suspension of boehmite-like (aluminium oxyhydroxide) hydrated nano/micron size crystals in loose aggregates • The products have v...
-
Product DYNO®-MILL KD
Grinding container volume of 6 to 600 liters.
The DYNO®-MILL KD is an agitator bead mill with horizontal grinding container. Specially designed agitator discs, mounted symmetrically on a shaft, transfer the energy required for wet milling and dispersion to the spherical grinding beads. An external pump ...
-
Product VET-SAP
VET-SAP® is a veterinary vaccine adjuvant derived from a semi-purified saponin fraction of the Quillaja saponaria tree bark. It is formulated with the purest saponin adjuvant on the market, boasting 90% saponin purity.
Customers can expect consistent saponin purity and profile in every batch, ...
-
Product API-Dextran 20
Product name: Dextran 20
Normative Mw: 20000
CAS NO: 9004-54-0
Appearance: White Crystal powder
Specification: BP, EP, USP, CP
Documentation: GMP, DMF, EU written confirmation
Application: Blood Volume Expander, Organ...
-
Product INACTIVATE - I22
INACTIVATE / I22 is a device for the inactivation of pathogens & cells of any kind. By low energy electron irradiation (LEEI) the genetic material of the pathogens & cells is reliably destroyed. The I22 device is suitable for research purposes and small industrial batches. Example use-cases are: Vaccine ...
-
Product DYNO®-MILL UBM (Universal Bead Mill)
Grinding container volume of 0.5 to 100 litres.The name DYNO®-MILL UBM speaks for itself: Universal Bead Mill. It was developed for universal use. The new generation of WAB agitator bead mills covers the entire range from dispersion to ultra-fine grinding. It is suitable for grinding bead diameters fr...
-
Product Group A and Group C Meningococcal Conjugate Vaccine (CRM197) Menphecia®
• China's first bivalent meningococcal conjugate vaccine using CRM197 vector • Provides protection against two serotypes (group A and group C) • Safer and better

-
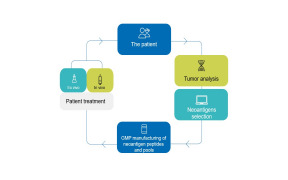
Product NEOANTIGEN PEPTIDES
Our Peptide-based Personalised Medicine Laboratory (PPM Lab) has been designed and equipped with state-of-the-art technology to meet the unique requirements of neoantigens production. A dedicated and experienced team of chemists and doctors ensures full control of the processes.
The implementati...
-
Product RAKSHARAB
Raksharab is recommended for immunization of dogs against rabies and contains inactivated rabies virus. The virus is propagated in BHK-21 Cell line, inactivated with an aziridine compound and concentrated. Single dose vial : 1 ml Multidose vial containing 5 doses : 5 ml Multidose vial containi...
-
Product Influenza Vaccine (Split Virion), lnactivated – Anflu®
Influenza Vaccine (Split Virion), lnactivated – Anflu®
Pandemic Influenza Vaccine, lnactivated, Adjuvanted – Panflu®
H1N1 Influenza A Vaccine (Split Virion), lnactivated – Panflu.1®
Influenza Vaccine (Split Virion), lnactivated, Quadrivalent – TetrAnflu®
Anflu®, preservative-free ...
-
Product Media for Vaccine Production
WakoVAC is FUJIFILM Wako Pure Chemical's Serum Free Cell Culture Media Series for Vaccine Production.The lineup includes for MDCK cells, Vero cells and Insect cells.A series of media with very high performance in cell growth. We are especially aiming to support vaccine manufacturing with our quality prod...
-
Product Fermenters
Fermentation is mostly extracellular activity and is brought with the help of enzymes released by the microorganisms. The Fermenter is designed to meet all process requirement for micro-organism to achieve maximum growth and productivity. Features:
• Fully automated or semi-automated models
• 21...
-
Product ABHAYRAB
Abhayrab is a purified Inactivated Rabies Vaccine prepared on Vero cells and is used for the prophylactic or post exposure immunization against Rabies. The vaccine is prepared using L.Pasteur 2061/Vero Rabies strain and is exported widely to 40 countries worldwide. Each pack consists of 1 dose vial along w...
-
Product Vaccines
Biomed has a range of vaccines with effectivnes proven in clinical studies.
Clodivac (Diphtheria and tetanus vaccine adsorbed, reduced antigen content)
Indications: For active immunization of adolescents and adults against tetanus and diphtheria
Active substance: Tetanus and diphther...
-
Product Combipack Of Snake Venom Antiserum
Premium Serums & Vaccines Pvt Ltd offers a wide range of products which includes combipack of snake venom antiserum. Content: it has indian cobra venom (naja naja), common krait venom (bungarus caeruleus), russell’s viper venom ( vipera russelii ), saw scaled viper venom (echis carinatus), cresol (preserva...
-
Product 23-valent Pneumococcal PolysaccharideVaccine
Covers a wider range of pneumococcal serotypesMore economical for low-and middle-income countries
Patented process
Great immunogenicity and safety
Prefilled Syringe
-
Product Trastuzumab Injection
[Dosage form]: Liquid for injection [Specification]: 150mg/vial; 440mg/vial (pipeline)
[Indication]: Indicated for Metastatic breast cancer, Early breast cancer, Metastatic gastric cancer.
[Administration]: Intravenous injection
[Shelf life]: 24 months
-
Product GMP quality control of biologics
CLEAN CELLS is a certified Pharmaceutical Company committed to deliver high-level quality services.
GMP service provider that is specialized in cell and virus banks manufacturing (MCB, WCB, MVS, WVS), biosafety testing, potency assays, bioprocess validation and development/validation &nb...
-
Product Human Serum Albumin
Human Serum Albumin for research and further manufacturing use.
Human Serum Albumin is widely used as an excipient in drug formulation, as a component of cell culture media, for drug delivery, cryopreservation of cells, vaccine manufacturing, or coating of medical devices; ultimately making it a...
-
Product Pharma Latch Hollow
The Pharma Latch Hollow is a sterile-packaged, single-use, disposable medical device intended for use with any liquid formulation approved per the intradermal route of administration.
The Pharma Latch Hollow is compatible with standard luer-lock and slip syringes and can inject high volume and...
-
Product QS-21
- Desert King QS-21 is the globally accepted human vaccine-quality benchmark.
- QS-21 is one of the world’s most powerful immunostimulants.
- QS-21 is derived from the bark of the Quillaja saponaria Molina tree using advanced HPLC separation technology for purification.
qq...
-
Product Biotest Human Serum Albumin
Human Serum Albumin for the biotechnology and pharmaceutical industry We offer a human serum albumin (HSA) product that complies with the regulatory requirements of multiple health authorities. It is manufactured in a GMP compliant, state-of-the-art manufacturing pla...
-
Product Trastuzumab Injection
[Dosage form]: Liquid for injection [Specification]: 150mg/vial; 440mg/vial (pipeline)
[Indication]: Indicated for Metastatic breast cancer, Early breast cancer, Metastatic gastric cancer.
[Administration]: Intravenous injection
[Shelf life]: 24 months
-
Product Nucleic Acid Delivery for Vaccines and Next-Gen Therapeutics
Avanti Polar Lipids, part of Croda Pharma, develops innovative lipids of unparalleled purity to solve the stability and delivery issues associated with mRNA for use in vaccines and next-generation therapeutics.
We develop and manufacture highly specialised lipids used in Lipid Nanoparticle (LNP) deliv...
-
Product Recombinant Human Basic Fibroblast Growth Factor for External Use
[Dosage form]: Freeze-dried powder [Specification]: 20,000IU/vial, 35,000IU/vial, 70,000IU/vial, 300,000IU/vial
[Indication]: Promote wound healing and can be used for chronic wounds (including chronic granulation wounds, ulcers and bedsores, etc.), fresh wounds (including trauma, surgical ...
-
Product Sterile Dosage Filling and Lyophilization
IDT Biologika GmbH provides a wide range of pharmaceutical services which includes sterile dosage filling in vials and pre-filled syringes. With a facility size of 3,600m2, this integrated pharmaceutical/ biological site houses more than 7 filling lines for the aseptic filling of syringes, vials, ampoules ...
-
Product Cell bank and virus seed characterization
As a world leader in biopharmaceutical testing services, we offer you a comprehensive range of biologics safety testing , including virology, cell and molecular biology, as well as microbiology and electron microscopy. As a result, we can help you to ensure product safety and meet your regulatory requireme...
-
Product 13-Valent Pneumococcal Polysaccharide Conjugate Vaccine
[Dosage form]: Suspension for injection [Specification]: 0.5 ml/dose
[Indication]: Protect infants over 2 months, young children, and adults against disease caused by the bacterium Streptococcus pneumonia.
[Administration]: Intramuscular injection
[Storage temperature (℃)]: ...
-
Product CordenPharma Lipids & Carbohydrate Platform
Your Expert Partner for Standard & Proprietary Lipids & Carbohydrates
• >> A specialized Lipids offering including custom and standard lipids (Glycero-Phospholipids, Sphingolipids, Phosphocholine, Pegylated, and Cationic Lipids). • >> Lipids for support of mRNA vaccine ...
-
Product Hepatitis A Vaccine (Human Diploid Cell), Inactivated - Healive®
• Hepatitis A Vaccine (Human Diploid Cell), Inactivated – Healive® Launched in 2002
Hepatitis A & Hepatitis B Combined Vaccine – Bilive®
Launched in 2005
Healive® is:
Prequalified by the World Health Organization.
Provides susta...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
.png)
.jpg)