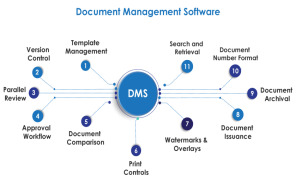
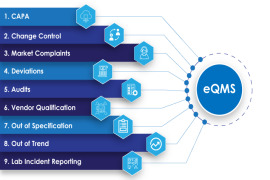
EasyFMD - Falsified Medicine Directive Software

Product Description
Quick Pharm Solution Limited

-
IE
-
2024On CPHI since
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
Quick Pharm Solution Limited

-
IE
-
2024On CPHI since
-
1 - 24Employees
Company types
Primary activities
More Products from Quick Pharm Solution Limited (2)
-
Product EU FMD Serialisation Partner Service
Our Serialisation Managed Service is a cost-effective solution to help Pharmaceutical Manufacturers, and Market Authorisation Holders achieve FMD compliance
-
Product Global Medicine Aggregation
Find out what is inside a case or pallet by scanning the code on the outside, without having to open the case to get the serial number of each individual pack-level product. Quick Pharm Solutions Global Medicine Aggregation aggregation is the solution!
Quick Pharm Solution Limited resources (2)
-
News FMD Serialisation Deadline Approaching for Italy and Greece. Are you ready?
The clock is ticking for Italy and Greece as the EU Falsified Medicines Directive (EU-FMD) deadline approaches in February 2025.
-
Video First ever pharmaceutical packs in Malta
Quick Pharm Solutions, in conjunction with our partners at Serialisation Malta (www.serializationmalta.com), are proud to announce that we have serialized the first ever pharmaceutical packs in Malta for the Maltese market.
This achievement is very timely given the current medicine shortages in Malta and across Europe.
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






































%20(002)-comp302826.png)