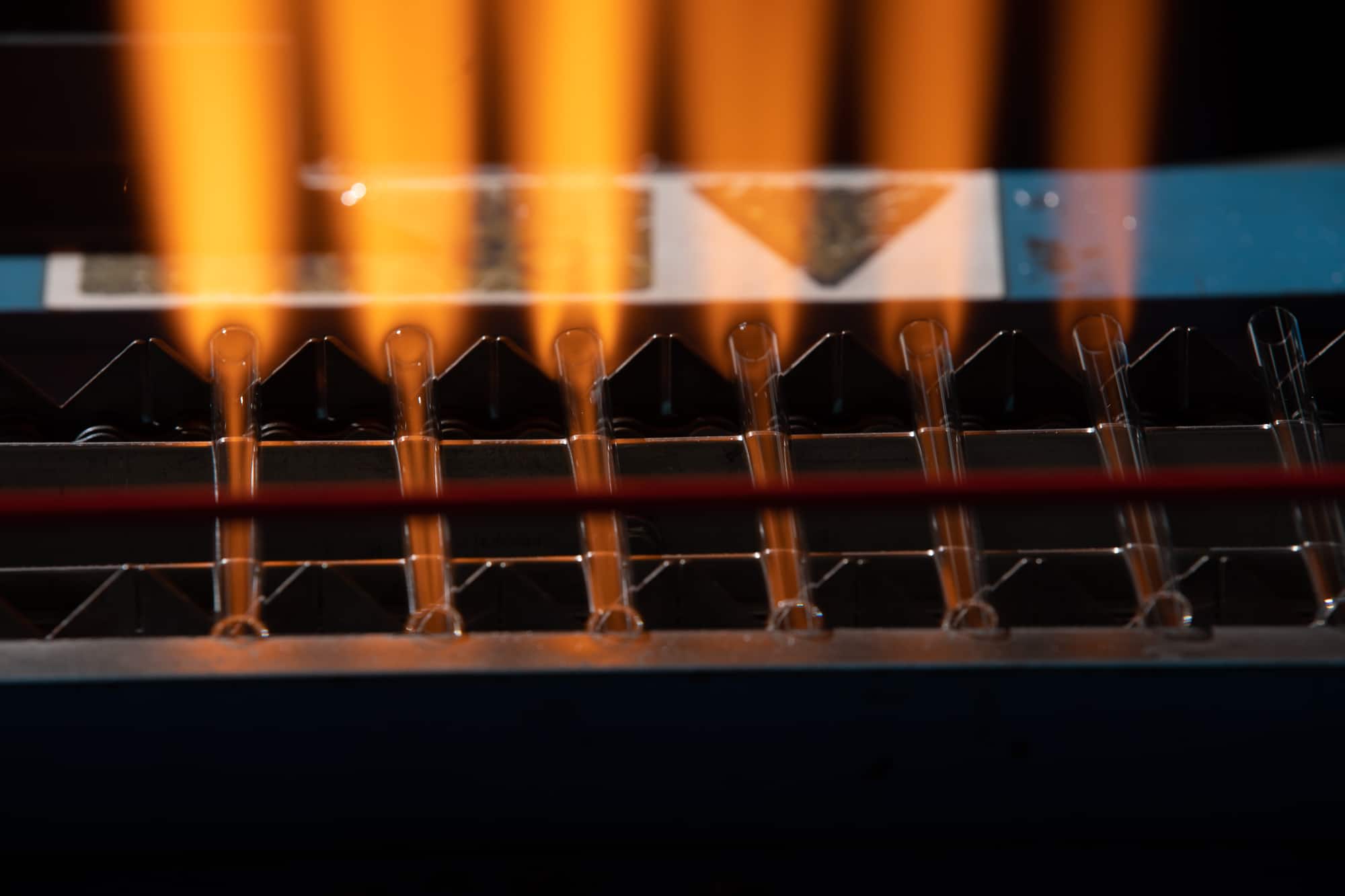
DWK Life Sciences Vial Manufacturing Capabilities
Product Description
DWK Life Sciences

-
GB
-
2020On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Categories
Specifications
DWK Life Sciences

-
GB
-
2020On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
More Products from DWK Life Sciences (4)
-
Product DWK Life Sciences Pipette Forming
DWK Life Sciences is an established glass pipette manufacturer to the Diagnostics, Healthcare, Homeopathy and Personal Care sectors across the globe. Utilizing DWK's extensive manufacturing and development capabilities, our flexible product offering includes standard and custom pipettes. Glass dropper pi... -
Product DWK Life Sciences Primary Packaging Solutions
DWK Life Sciences is a leading global manufacturer and supplier of glass and plastic primary packaging. Our Primary Packaging container closure systems are manufactured from the highest quality pharmaceutical-grade tubing designed specifically for pharmaceutical, biotech, diagnostic/IVD, and personal ... -
Product Stevanato Group EZ-fill® Platform
• Clean, depyrogenated, and sterilized ready-to-use (RTU) vials designed for pharmaceutical and contract manufacturing entities performing small to large batch aseptic parenteral packaging. • ISO 8362-1 2R, 4R, 6R, 10R, and 20R vials, manufactured from borosilicate type 1, 51 expansion glass, with Europe... -
Product DURAN® PURE Bottles and Closures
Duran Pure is a range of high-quality glass bottles and plastic closures for storage and transport of APIs, intermediates, excipients and media. The main application area is in sterile manufacturing of pharmaceutical and biotech products according to Good Manufacturing Practice (GMP).
DWK Life Sciences resources (4)
-
Brochure STEVANATO GROUP EZ-fill® - READY-TO-USE VIALS
Pre-sterilized Glass Vials Accelerate Workflows from Pipeline to Patient -
Brochure DURAN® PURE - THE API BOTTLE SYSTEM
Biotech & Pharmaceutical GMPIn-Process and Packaging Container -
Brochure DURAN® PURE - THE API BOTTLE SYSTEM
Biotech & Pharmaceutical GMPIn-Process and Packaging Container -
Brochure DURAN® PURE - THE API BOTTLE SYSTEM
Biotech & Pharmaceutical GMPIn-Process and Packaging Container
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance















(1)v2.jpg)








































































.png)
















.png)