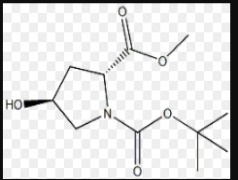
Drug substance production

Product Description
Biologics Manufacturing Company Inc.

-
CA
-
2024On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
Biologics Manufacturing Company Inc.

-
CA
-
2024On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from Biologics Manufacturing Company Inc. (4)
-
Product Analytical services
Our expertise spans across several critical areas:
Microbiology analysis • Microbial Identification (Micro ID) • Bioburden Testing • Endotoxin Testing Utilities Testing (Microbiology and Chemistry) • Water System Testing • Clean Steam Testing • Air Quality Monitoring Raw Ma... -
Product Fill and Finish
Formulation, Filtration, Filling, Visual Inspection, Labelling, Packaging -
Product Secondary packaging
The BMC is currently able to answer your needs for packaging of: - Vials 2R Glass or SiO2 Plastic with labelling, cartonning and case packing.
- Vials 10R Glass or SiO2 Plastic with labelling, cartonning and case packing.
The BMC has also the potential of serving yo... -
Product Technology Transfer
Maximize the production capabilities through a de-risking process with our comprehensive technology transfer services for engineering batches, process performance qualification (PPQ) batches, and commercial batches of vaccine antigens, including viral vectors, protein subunits, and virus-like par...
Biologics Manufacturing Company Inc. resources (1)
-
Video Biologics Manufacturing Centre Company Presentation
Biologics Manufacturing Centre Company Presentation
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






























































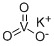













.jpg)