Drug Products
Product Description
CARBOGEN AMCIS

-
CH
-
2015On CPHI since
-
1Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
CARBOGEN AMCIS

-
CH
-
2015On CPHI since
-
1Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from CARBOGEN AMCIS (7)
-
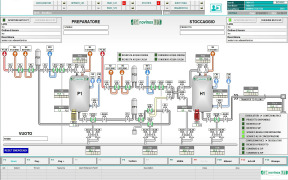
Product Analytical Services
The complete integration of analytical sciences into the process of API development is fundamentally understood at CARBOGEN AMCIS. Our scientists, technique ranges, systems (including a fully validated LIMS) and procedures facilitate a full understanding of the unique characteristics of the complex molecul... -
Product Clinical API Development
CARBOGEN AMCIS has a DNA in API development, stretching back over 30 years. Our API development teams are selected for their passion for science and experience in the application of phase-appropriate efforts to solve challenging chemistry issues. From taking a promising molecule from our clients' medicinal... -
Product Commercialization Services
CARBOGEN AMCIS has a track record, extending over 25 years, helping our customers transition their molecules from process development through validation and ultimately into commercial status. A range of services backed up with fully integrated support functions has been developed to facilitate and smooth t... -
Product Highly Potent API
CARBOGEN AMCIS has state of the art facilities and controls to handle both drug substance and drug product of the highest potency and toxicity, under cGMP. Our dedicated containment facilities use a containment methodology, utilizing barrier isolation technology and Rapid Transfer Ports (RTPs), combined wi... -
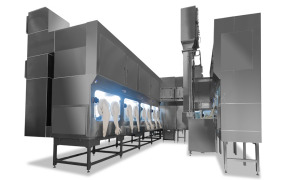
Product Sterile Drug Product services
CARBOGEN AMCIS offers Sterile Drug Product services, specializing in the formulation, development, and manufacturing of injectable products. With capabilities in lyophilization, sterile filling, and handling highly potent APIs, the company ensures aseptic processing for clinical and commercial supply. ... -
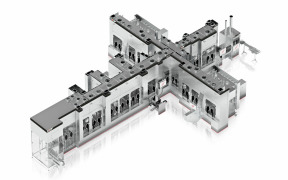
Product Drug Substance Services
CARBOGEN AMCIS provides comprehensive Drug Substance services, offering expertise in process development, scale-up, and manufacturing of active pharmaceutical ingredients (APIs). With state-of-the-art facilities, the company specializes in high-potency compounds, including cytotoxics, and delivers solu... -
Product Specialties Services and Products
Specialty manufacturing and services provided by CDMOs address the diverse needs of customers in various therapeutic, nutraceutical and dietary supplement areas, as well as in the pharma, personal care, and cosmetics industries.
With over 70 years of experience, CARBOGEN AMCIS Veenendaal...
CARBOGEN AMCIS resources (4)
-
News CARBOGEN AMCIS Secures GMP Certification After Successful ANSM Inspection for its Aseptic Drug Product Manufacturing Site
CARBOGEN AMCIS is pleased to announce the successful completion of the first inspection by the French regulatory authority, ANSM (Agence nationale de sécurité du médicament et des produits de santé), at its state-of-the-art sterile drug product manufacturing site in Saint-Beauzire, France.
The inspection, conducted from January, 20th to January, 24th, follows the site’s opening in February 2023. This is the first inspection of a routine process by ANSM to ensure compliance with Good Manufacturing Practices (GMP). As a result, CARBOGEN AMCIS has been granted a GMP certificate for the Saint-Beauzire, France. -
Video CARBOGEN AMCIS Secures GMP Certification
CARBOGEN AMCIS is pleased to announce the successful completion of the first inspection by the French regulatory authority, ANSM (Agence nationale de sécurité du médicament et des produits de santé), at its state-of-the-art sterile drug product manufacturing site in Saint-Beauzire, France.
CARBOGEN AMCIS has been granted a GMP certificate for the Saint-Beauzire facility, marking a significant milestone in the site's successful start-up. The certificate allows for the manufacture and release of clinical and commercial sterile drug products. The site received authorization from the ANSM to manufacture, test and release drug products from February 2023 and since then has been working under this authorization with the first clinical batch released in January 2024. -
News CARBOGEN AMCIS announces major investments of more than CHF 100 million in Switzerland and France
Switzerland-based CARBOGEN AMCIS, a pharmaceutical process development and Active Pharmaceutical Ingredient (API) and drug products manufacturing company, announced today new expansion plans in Switzerland and France. -
Brochure Company Profile
Dowload our brochure and learn more about our offers.
Drug development to Commercialization services for pharmaceutical and biopharmaceutical industries at all stages of drug development
Your CDMO from Drug Substance to Drug Product
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




































































.jpg)









































.png)