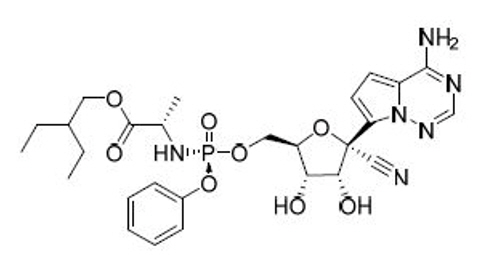
DELOS FORMULATION TECHNOLOGY
.png)
Product Description
Nanomol Technologies S.L.

-
ES
-
2022On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
Nanomol Technologies S.L.

-
ES
-
2022On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
More Products from Nanomol Technologies S.L. (2)
-
Product CONSULTING SERVICES ON PARTICLE CHARACTERIZATION
Consulting services which create value by combining our expert scientific background with highly qualified staff, equipments and facilities, to deliver advanced particle characterization studies such as:
- Development validation and transfer of analytical methods
- Comparative... -
Product CONTRACT PARTICLE ANALYSIS - Routine Analysis and R&d Studies
Analytical services for Drug Substance & Drug Product advanced particle characterization.
GMP services which include: • Particle Size Distribution (PSD) by Laser Diffraction • Morphological and Particle Size Parameters by microsocopy • NanoParticle Size and Concentration by MADLS • Z-Potentia...
Nanomol Technologies S.L. resources (4)
-
News Pilot Plant for the Production of DELOS Nanoformulations
A significant milestone is on the corner!
We are pleased to announce that our GMP pilot plant for the production of DELOS nanoformulations is almost ready! -
Brochure Particle Characterization Unit - CONTRACT PARTICLE ANALYSIS
Analytical services for Drug Substance & Drug Product advanced characterization. Nanomol Technologies provides specialized physicochemical characterisation services for the pharma and biotech industry. We are a team of experts in analysing Critical Quality Attributes (CQA) of particulate materials. -
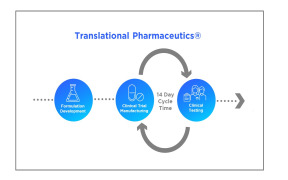
Brochure DELOS Technology Unit - DELOS FORMULATION TECHNOLOGY
Development and Manufacturing of nanomedicines and tailored drug delivery systems using patented nanoparticles. Nanomol Technologies provides expert and propietary nanoparticle formulation technology from early preclinical development up to the clinical phase for the pharma and biotech industry. Our qualified team holds a vast knowledge in particle engineering and nanomedicine development. -
Brochure Studies for the nanocharacterization of drug substances, drug products and ingredients.
We offer complete analytical nanocharacterization studies in order to properly define critical quality attributes (CQAs) of nanopharmaceuticals, drug delivery systems, nanoparticulate materials, cosmetic and food ingredients.
We are experts in the development of analytical methods at nanoscopic level. We can provide service packages to design and implement analytical tools which enable to measure and monitor the following CQAs and specifications.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






_scaled.png)















.jpg)






















![NexArni 50 [Valsartan with Sacubitril Tablets]](/46/product/129/06/57/picture.jpeg)