- Home
- AZ Pharmaceutical Group Limited
- DAPTOMYCIN FOR INJECTION, 0.5g
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
DAPTOMYCIN FOR INJECTION, 0.5g

Product Description
For treatment of bloodstream infection (bacteremia) associated with right
infective endocarditis caused by Staphylococcus aureus(including methicillin
sensitivity and methicillin resistance).
AZ Pharmaceutical Group Limited

-
CN
-
2017On CPHI since
Contact info
-
-
-
No. 7 Huiling Road,, Torch Hi - tech Development Zone, 528437, Zhonongan, Guangdong, China
Categories
Specifications
- Selling PointsImmune Health; International Approvals/Standards
- Supplied fromChina
- Measured Inpiece
Sales Markets
- Middle East Region (e.g. UAE)
- Oceania
- North America (USA, Canada)
- Africa
- Central America (e.g. Mexico)
- East Asia (e.g. China, Japan, Korea)
- Europe - EU countries
- Europe - non EU (e.g. UK, Russia, ex-CIS countries)
- South America (e.g. Brazil, Colombia)
- South Asia (e.g. India, Pakistan, Sri Lanka)
- South East Asia (e.g. Thailand, Philippines, Singapore)
AZ Pharmaceutical Group Limited

-
CN
-
2017On CPHI since
Contact info
-
-
-
No. 7 Huiling Road,, Torch Hi - tech Development Zone, 528437, Zhonongan, Guangdong, China
More Products from AZ Pharmaceutical Group Limited (2)
-
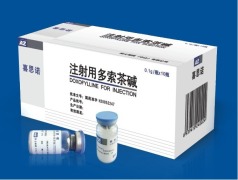
Product DOXOFYLLINE FOR INJECTION, 0.1g
Suitable for Dyspnea due to bronchial asthma, chronic bronchitis and other bronchospasm . -
Product LEVOFLOXACIN MESYLATE FOR INJECTION
strength 0.2g, 0.3g
It suitable for moderate or severe infections caused by sensitive bacterial, including respiratory system infection, acute bronchitis, acute exacerbation of chronic bronchitis, diffuse bacterial bronchitis, bronchiectasis, pneumonia, tonsillitis,etc.
Recommended Products
-
Product Development Sciences
Our development laboratories are well equipped for conducting a diverse range of experiments. These encompass formulation development, cycle design and process refinement, as well as evaluating finished product. Ease of scale-up is accomplished by completing process development studies within a pilot scale...
-
Product Containment Systems for API Pilot Plant - Includes: Filtration, Milling,...
Production and handling of HPAPI or toxic products (in gas or powder form) need the total product separation from the environment to protect operators. Negative pressure regime is maintained within the closed volume of the isolator. Turbulent flow is generally adopted.
Operator access to isolator inside...
-
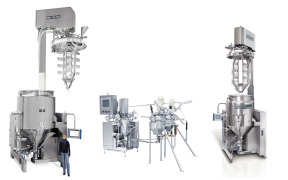
Product Process Plant Master Plant
The IKA Master Plant homogenizing and emulsifying plant is a universal mixing plant developed for the production of emulsions and suspensions in the pharmaceuticals industry in particular, but also in the food, beverages, cosmetics and chemical industries. The plant is GMP-compliant and guarantees ...
-
Product Gelucire 59/14
Gelucire® 59/14 is a mixture of lauroyl PEG-32 glycerides and PEG 6000 and has surfactant properties. It is a self-emulsifying excipient used for oral bioavailability enhancement of poorly water-soluble drugs, thanks to its high solubilizing capacity.
-
Product Grindeks FDF portfolio
Please see Grindeks up-to-date Final Dosage Form (FDF) product portfolio, including pipeline products, in the link attached. Dossiers are harmonized according to ICH and EMA requirements. https://grindeks.com/en/products/final-dosage-forms/ -
Product SOLUBLE TECHNOLOGY
Soluble formulations are solid immediate release forms that need to be dissolved in water with rapid stirring at the time of administration. They yield an oral solution or suspension, depending on API solubility. In both cases the API is ready for absorption in the gastrointestinal system resulting in a fa...
-
Product Auxiliar y Servicios DELSAZ
Auxser is the leader in maintenance and optimization of lyophilization processes. With more than 15 years of experience working with the main pharmaceutical and food companies, Auxser has a team of professionals and specialized media in the maintenance, repair and optimization of lyophilizers in Spa...
-
Product CALMAHYAL - A low cost Hyaluronic Acid for all markets - Lubricant Ophth...
Hyaluronic acid for the relief of dry eye
Indications.Lubricating ophthalmic solution indicated for the symptomatic treatment of dry eye syndrome. Also indicated forcorneal protection as a tear substitute.
General practioctioners & Specialists
Medical Device class IIb q...
-
Product Formulation Development
MedPharm has more than 20 years’ experience developing topical and transdermal formulations. We can begin as early as API screening and support pre-formulation activities, lead formulation selection, formulation optimization and reverse engineering. We have had a role in taking over 55 products to mark...
-
Product Cisplatin
CISPLATIN 50 mg lyophilized vials – Several types of cancer (testicular, ovarian…). Authorized EU-CTD Dossier
-
Product Vigor Forte
Lack of appetite, growth, child’s asthenia in the convalescences of infectious or debilitating diseases.
DOSAGEfrom 2 to 6 years: 1 vial a day preferably in the morning.
INGREDIENTSIn the filler cap: Freeze-dried Royal Jelly 100mg.In the vial: Fresh Royal Jelly 300mg, Acacia hone...
-
Product GINESTRA®
GINESTRA is a medical device class IIA in the form of vaginal tablets to be used in all cases of temporary alteration of the balance of the urovaginal ecosystem.
-
Product Syntegon SVP Process System
The highly modular and standardized system solution providing the highest degree of flexibility in both the layout of the customer’s room concept and the configuration of the production units themselves. Over 70 pre-tested functional modules are available to process batch sizes from 13 to 3,000 liters.
-
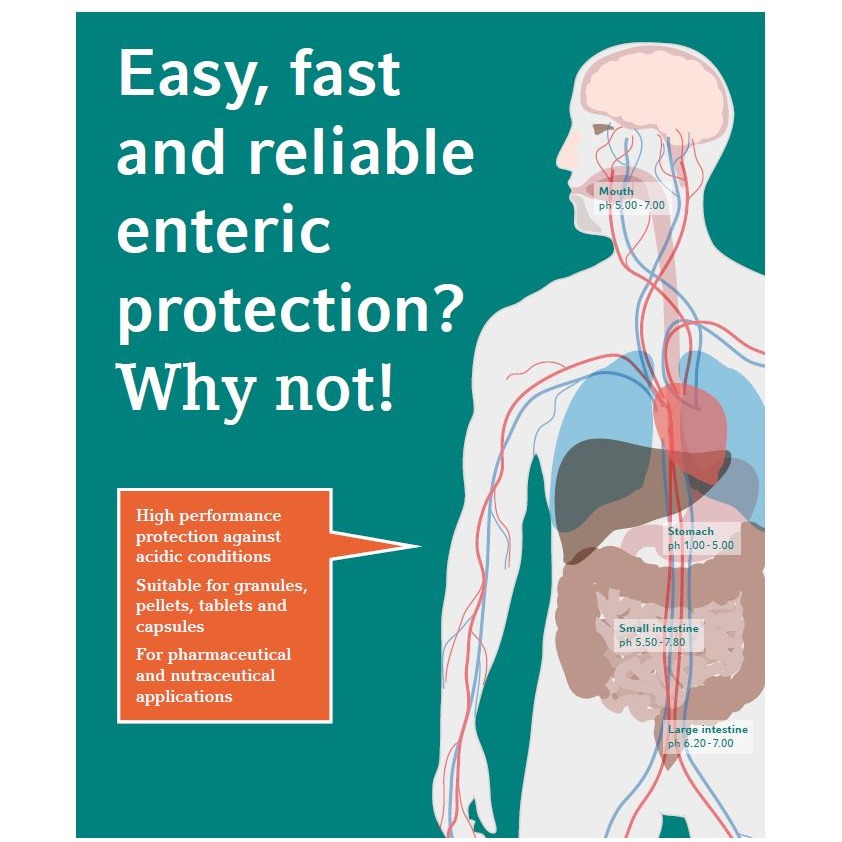
Product Functional film coating
Easy, fast and reliable enteric protection? Why not! - High performance protection against acidic conditions. - Suitable for granules, pellets, tablets and capsules. - For pharmaceutical and nutraceutical applications.
-
Product Lyophilisation
Famar offers lyophilisation services. It can perform aseptic filling and lyophilisation in vials from 2ml to 20ml.
Contact us for more information!
-
Product HiCel HFS: Premix for moisture sensitive or poor soluble API
HiCel HFS , a high functionality excipient , it is a combination of three pharmacopeial excipients and made by co-processed technology. HiCel HFS contains 10% Mannitol , 1.5 % colloidal silicon dioxide and 88.5% Microcrystalline cellulose.
HiCel HFS provides excellent...
-
Product Somatropin for injection USP 16 IU – Shreetropin
A human growth hormone drug for dwarf child
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Somatropin for injection USP 16 IU-Somatropin.
• Use: Treatment for children with growth failure due to inadequate secretion of endogenous growth...
-
Product Dexamethasone Sodium phosphate ®
EIPICO offers a wide range of corticosteroids which includes dexamethasone sodium phosphate ®, dexamethasone sodium as phosphate 8mg /12ml. It belongs to systemic hormones category. Contact us for more information.
-
Product Life cycle management of branded products
Our ‘life cycle management’ development services are focused on maximizing the value of your drug during its product life cycle. Typically, we are asked by pharmaceutical and biotech companies to develop new product formulations of branded drugs to anticipate on patent expiries, generic competition...
-
Product Lifetech™ SpinChem® MagRBR
A magnetic, rotating bed reactor, pre-packed with a variety of Lifetech™ ECR resins. Designed for parallel laboratory development and reaction screening in 5 – 100 mL volumes enabling easier and faster screening and optimization of biocatalysis production processes. Developed in collabortion with SpinChem.
-
Product Freeze drying
The value proposition generated through two sites specialized in freeze drying is unique, from manufacturing process optimization to producing freeze dried characterized and documented vials, allowing our clients to diff erentiate on their own markets. We recently invested 7 million euros to increase capac...
-
Product Formulation & Development
Formulation Product development team includes experienced Scientists who continuously work for the development of New Drug Product, Dietary Supplement, Generic Drug Products, Novel Drugs Delivery System (NDDS), New fixed dose combination products and Herbal Cosmetics & Herbal Products.
We offer...
-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Formulation Development
Quotient Sciences has almost 30 years of experience developing a breadth of pharmaceutical formulations across a range of indications. Our innovative approach to formulation development integrates drug product development with clinical evaluation, combined with our experience with over 1,000 molecules at a...
-
Product CordenPharma Sterile Powder Lyophilized Vials
CordenPharma has extensive experience in sterile contract manufacturing with a wealth of lyophilization expertise. In particular, the optimization of the freeze drying process as it relates to cycle times represents a critical attribute for complex parenteral product development. CordenPharma experts suppo...
-
Product Vitarojal® classic 1000 mg liquid food supplement with royal jelly and h...
Apipharma d.o.o. Offers a wide range of products which includes vitaroja.l 1 vial contains 333.3 mg of lyophilized royaljelly which is the equivalent to 1000 mg offresh royal jelly.Vitarojal® classic 1000 mg contain royaljelly which is known for its biostimulating properties.Each vial contains 1000 mg of r...
-
Product R&D and Industrialization
We specialise in research and development in the ophthalmic field for multinationals, Italian as well as international pharmaceutical companies. For them, we develop, fine-tune and manufacture, in aseptic conditions, pharmaceuticals and sterile medical devices for topical use in differen... -
Product Aseptic filling and visual inspection
For over 40 years, our fill and finish services have helped to keep the world supplied with injectable therapies, many of which are lifesaving. Our partnerships with customers start in the first steps of clinical manufacturing through commercial-scale filling and beyond, including management throughout the...
-
Product Lyophilization
Developing and optimizing lyo cycles to support clinical and commercial programs
-
Product Injectable Formulation Development & Fill-Finish Services
WuXi STA offers a wide range of tailored services and solutions for clients developing injectable formulations. Form types include solution, emulsion, sterile powder (lyophilized) and liposome. Our offering includes formulation development, performance analysis and characterization testing, and fill/...
-
Product Early stage formulation Development & Process Design
We have extensive experience in solving complex formulation challenges. Our experts can apply our extensive range of proprietary technologies for immediate (IR) and modified (MR) / sustained, slow (SR) / controlled (CR) / extended (XR, ER) / pulsed / timed release as well as enteric and gastro reten...
-
Product Liposomal Drug Delivery Technologies Development Support and Analysis
Liposomal drug product characterisation according to the FDA CMC guidance to support your new drug application or biologics license applications. Including:
• Physicochemical parameters determined to be CQAs (e.g. particle size, size distribution, zeta-potential and physical stabi...
-
Product Complex Semi-synthetic APIs
Micafungin Sodium, Anidulafungin-Fructose, Dalbavancin, Everolimus, Sarecycline, Midostaurin etc.
-
Product Aseptic Fill Finish
We offer two convenient fill-finish options for parenteral drug products: prefilled syringe and vials. As a leading pharmaceutical partner, we can provide all necessary elements of delivering high-quality parenteral products for clinical and commercial needs.
AbbVie will leverage its know...
-
Product GALENIC DEVELOPMENT
TECNALIA, experts in Pharmaceutical Development, Scale-up & Pilot Batches Manufacturing, Clinical Trials and Contract Manufacturing
GALENIC DEVELOPMENT
• Preformulation studies.
• Design and galenic develop...
-
Product Freeze-drying
Freeze-drying, also known as lyophilisation, is frequently used to develop novel pharmaceuticals, biomaterials and a range of soft and hard tissues. EMCM can offer GMP approved freeze-drying services to its customers for a wide variety of products and production scales.
-
Product Bioequivalence tests for topical drugs
We are a GLP lab specialist in vitro permeation testing, including the bioequivalence tests under the EMA draft guideline. We support pharmaceutical companies through bioequivalence studies. Visit our booth and find out more about our R&D dedicated to this topic.
-
Product Spray Dryer
GEA Niro pharmaceutical spray dryers from R&D to large production scale. Come and discuss your Pharma Spray Drying project with us or learn more about our pharma spray drying technology. Welcome!
-
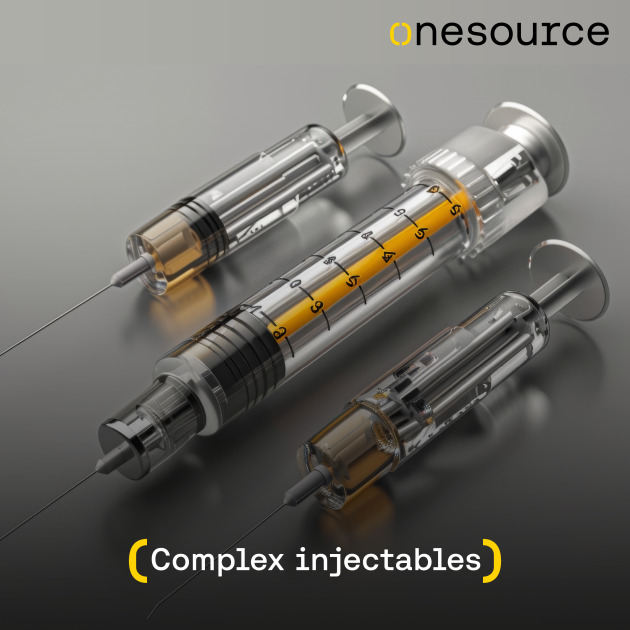
Product Complex injectables
25+ years of experience in complex formulation development, including ready to-use specialty products and novel drug delivery systems
Proven expertise in niche and complex dosage formats (single and dual-chamber bag, Lyo-vials, PFS, cartridges) to address hospital needs and improve patient experienc...
-
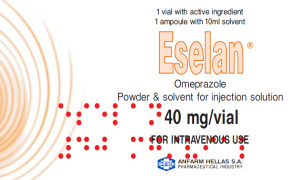
Product Omeprazole Ps.Inj.Sol 40mg/vial (EU CTD Available)
Omeprazole 40mg/vial Powder for Solution for Injection Reference Product: Losec® / Astra Zeneca
Description: Omeprazole belongs to the ATC category of Proton-pump inhibitors which is a group of drugs whose main action is a pronounced and long-lasting reduction of stomach acid production.Due t...
-
Product SAFFROX
100% Natural Multifunctional Antidepressant Saffrox™ is a new 100% natural advanced antidepressant composed of Saffron, turmeric, magnesium and vitamins. Studies shows efficacy same as Prozac(1) a Selective Serotonin Reuptake Inhibitor (SSRI), or Imipramine(2) but without the side effects of the ...
-
Product Solifenacin Succinate, Ibandronate Sodium, Sodium Alendronate, Zoledroni...
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and a diverse range of branded generic Finished Dosage Forms (FDFs) at its cGMP certified state-of-the-art manufacturing facilities in India, with a global presen...
-
Product ORODISPERSIBLE FILMS (ODF)
Orodispersible films (ODF) constitute an innovative oral drug delivery system.
This dosage form is placed on patient’s tongue or oral mucosal tissue and it rapidly dissolves to release the API for mucosal and sublingual absorption. As the film dissolves, the drug enters the blood strea...
-
Product Pharmaceutical Development - Early Phase
Almac’s experienced formulation scientists can develop a range of oral dose formulations for early stage clinical trials.
With both non-GMP and GMP facilities, flexible and efficient solutions are provided to develop a fit-for-purpose formulation for your early phase clinical trial.
...
-
Product Epsilon 2-6D LSCplus
Martin Christ Gefriertrocknungsanlagen GmbH offers a wide range of products which includes Epsilon 2-6D LSCplus. Features: it is a general-purpose, high-performance laboratory and pilot unit for drying solid or liquid products in vials, ampoules, tubes, other glass containers or dishes. Contact us for more... -
Product RheaLyo™ GMP-Flex
A GMP-compliant Continuous Freeze-Drying Production Line allowing aseptic processing and integration in the entire manufacturing chain of the sterile product.
Key features: Aseptic processing, Integrated PAT, Modular approach, Proven technology, Model-based design, High flexibility/production effici...
-
Product Co-Processed excipients for ODT (GRANFILLER-D®, HiSORAD™, SmartEx™)
Your benefits
- Ready-to-use mixture (filler, disintegrant, optionally taste masking)
- fast API release
- co-processed excipients with improved properties compared to physical mixtures
Applications
- Orally dispersible tablets (O...
-
Product Thermal characterization of the product
Having information on the thermodynamic behavior our formula is critical to identify the critical product temperatures. At Comser, we have the following technology to characterize the product:
Freeze Drying Microscope (FDM)
Differential Scanning Calorimetry (DSC)

Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















.jpg)