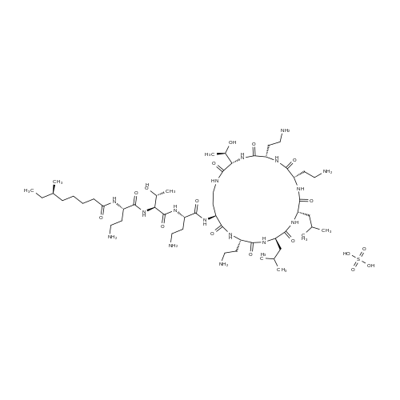
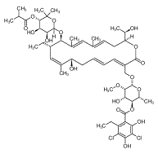
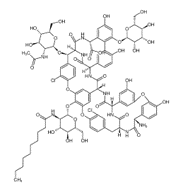
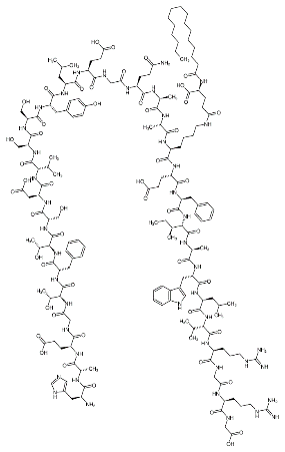
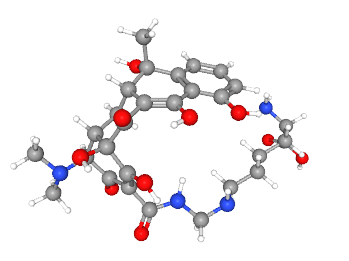
Dalbavancin

Product Description
BrightGene Bio-Medical Technology Co.,Ltd.

-
CN
-
2015On CPHI since
Specifications
BrightGene Bio-Medical Technology Co.,Ltd.

-
CN
-
2015On CPHI since
More Products from BrightGene Bio-Medical Technology Co.,Ltd. (49)
-
Product Trabectedin
• Status: Validated • Patent Expiration: ODE:2022.10.23;NCE:2020.10.23 • Usage: Oncology -
Product Alvimopan
• Status: Pre-validation • Patent Expiration: US:2022.11 • Usage: Intestinal Ostruction -
Product Posaconazole(Form Ⅰ)
• Status: DMF No.:032346 • Patent Expiration: Off-patent • Usage: Antifungal -
Product Posaconazole(Form Ⅲ)
• Status: DMF No.:030886 • Patent Expiration: Off-patent • Usage: Antifungal -
Product Sugammadex Sodium
• Status: DMF No. :033904 • Patent Expiration: US:2021.01.27; CN:2020.11.23 • Usage:Selective relaxant binding agent -
Product Tenofovir alafenamide,TAF
• Status: Validated • Patent Expiration: CN:2021.7;US:2032.8 • Usage: AntiHBV -
Product Oseltamivir phosphate
• Status: Validated and DMF ready • Patent Expiration: Off-patent • Usage: Anti-infection -
Product Doramectin
• Status: Commercial • Patent Expiration: Off-patent • Usage: Veterinary -
Product Eldecalcitol
• Status: Pre-validation • Patent Expiration: Off-patent • Usage: Osteoporosis -
Product Emodepside
• Status: Pilot • Patent Expiration: Off-patent • Usage: Veterinary -
Product Ferric carboxymaltose
• Status: Pre-validation, PV will be done in Oct • Patent Expiration: US:2027.03.04; • Usage: Iron Supplement -
Product Ferric citrate hydrate
• Status: Pilot • Patent Expiration: Off-patent • Usage: Iron Supplement
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
























































%20-%20copia-comp247805.jpg)