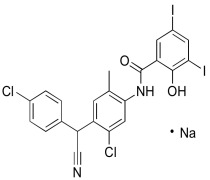
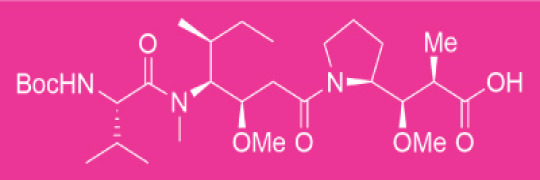
Crystallization

Product Description
J-Star Research Inc.

-
US
-
2017On CPHI since
Specifications
J-Star Research Inc.

-
US
-
2017On CPHI since
More Products from J-Star Research Inc. (2)
-
Product Analytical services
J-star research inc offers services which includes analytical services. Features: it includes all of the analytical needs for process development, glp studies and gmp campaigns. It is not limited to, method development, method verification / validation, reference standard certification, release testing, an... -
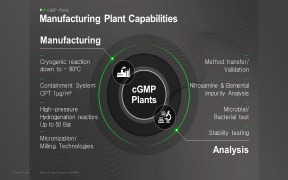
Product Cgmp synthesis and manufacturing process
J-star research inc offers services which includes cgmp synthesis and manufacturing process. Features: it is achieved through focus on quality systems management. A dedicated team of analytical chemists is there to ensure that intermediates and api manufactured materials are properly evaluated and conform ...
Recommended Products
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




































.jpg)