CORTIPOL 40 MG I.M./ I.V. POWDER AND SOLVENT FOR PREPARING INJECTION/INFUSION SOLUTIONS

Product Description
Polifarma Ilac

-
TR
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Polifarma Ilac

-
TR
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Polifarma Ilac (54)
-
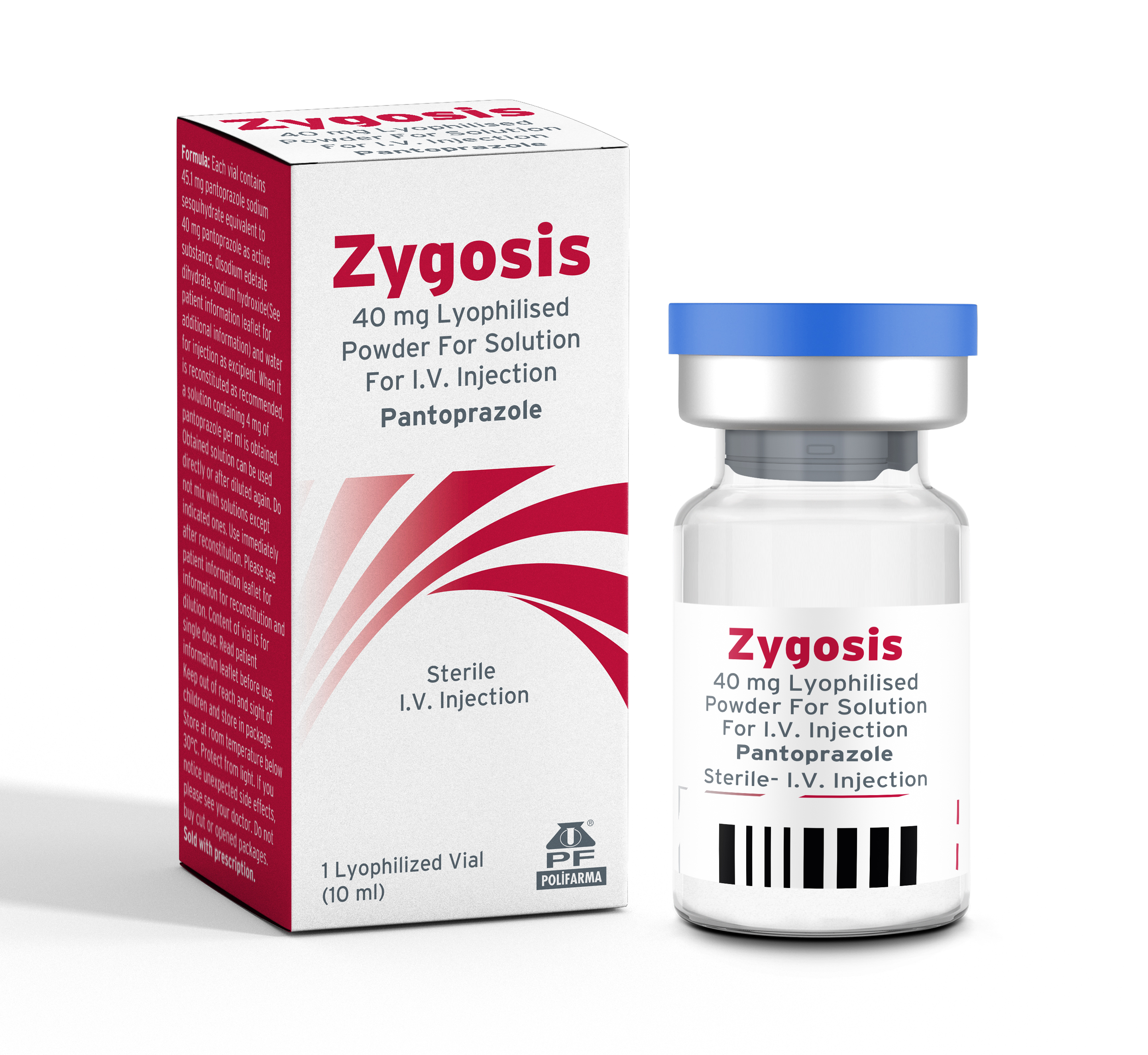
Product ZYGOSİS 40 Mg I.V. lyophilized powder to prepare solution for injection - 1 Vial
Active Ingredient: PantoprazoleTherapeutic Area: Proton Pump Inhibitor -
Product ANESED-R 0,5 MG/5 ML SOLUTION FOR I.M./I.V. INJECTION
Flumazenil is indicated for the complete or partial reversal of the central sedative effects of benzodiazepines. Therefore, it is used in anesthesia and intensive care in the following indications. In anesthesia: termination of general anesthesia using benzodiazepines for induction and maintenance. Rev... -
Product ASİMPLEX 250 MG LYOPHILIZED POWDER FOR SOLUTION FOR INFUSION
Herpes simplex infections in immunosuppressed patients: ASİMPLEX is indicated for the treatment of initial and recurrent cutaneous and mucosal herpes simplex (HSV1 and HSV2) in immunocompromised individuals and immunosuppressed patients. In initial infection of genital herpes: ASİMPLEX is indicated for ... -
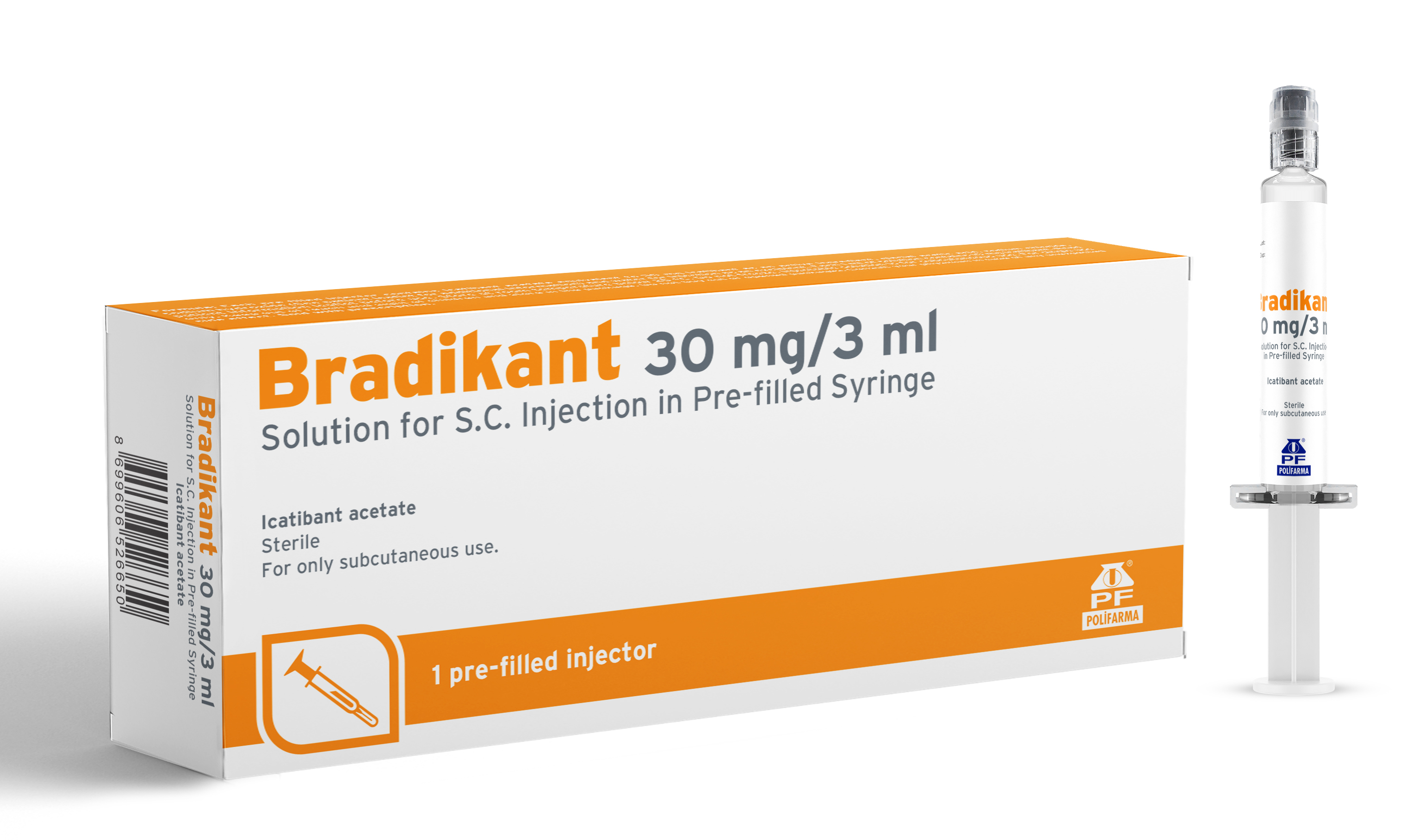
Product BRADİKANT 30 MG/3 ML SOLUTION FOR S.C. INJECTION IN PRE-FILLED SYRINGE
BRADİKANT is indicated for symptomatic treatment of acute attacks of hereditary angioedema (HAE) in adults, adolescents and children aged 2 years and older, with C1- esterase-inhibitor deficiency. -
Product CİPROPOL 2 MG/ML SOLUTION FOR I.V. INFUSION
In the presence of acute bacterial sinusitis, uncomplicated urinary infection and alternative treatment options, it should not be used due to the risk of serious side effects. In these indications, it can only be used with the approval of an infectious diseases specialist if it is proven with an antibio... -
Product CORTIPOL 20 MG I.M./ I.V. POWDER AND SOLVENT FOR PREPARING INJECTION/INFUSION SOLUTIONS
CORTİPOL is indicated to treat any condition in which rapid and intense corticosteroid effect is required such as: Endocrine disorders: Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; synthetic analogs may be used in conjunction with mineralocorticoi... -
Product DEKSTOMİD 200 MCG/2 ML CONCENTRATED SOLUTION FOR I.V. INFUSION
DEKSTOMİD is indicated for the sedation of patients who are intubated and mechanically ventilated since the beginning of their treatment at intensive care units. DEKSTOMİD should be administered as an infusion continuously for a maximum of 24 hours -
Product ENOPARİN 10000 ANTİ-XA IU/1 ML
ENOPARİN 10000 ANTİ-XA IU/1 ML -
Product ENOPARIN 2000 ANTİ-XA IU/0,2 Ml
ENOPARIN 2000 ANTİ-XA IU/0,2 Ml -
Product ENOPARİN 4000 ANTİ-XA IU/0,4 ML
ENOPARİN 4000 ANTİ-XA IU/0,4 ML -
Product ENOPARİN 6000 ANTİ-XA IU/0,6 ML
ENOPARİN 6000 ANTİ-XA IU/0,6 ML -
Product ESSİUM 40 MG POWDER FOR SOLUTION FOR I.V. INJECTION/INFUSION
Each vial contains 42,5 mg esomeprazole sodium equivalent to 40 mg esomeprazole.
Polifarma Ilac resources (2)
-
Video Corporate Introductory Video
Polifarma's Corporate Video -
Brochure Product list
Product list
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance