CordenPharma Lipids & Carbohydrate Platform

Product Description
Corden Pharma International GmbH

-
DE
-
2015On CPHI since
-
4Certificates
-
1000 - 4999Employees
Company types
Categories
Specifications
Corden Pharma International GmbH

-
DE
-
2015On CPHI since
-
4Certificates
-
1000 - 4999Employees
Company types
More Products from Corden Pharma International GmbH (32)
-
Product CordenPharma's Early-Phase Peptide Centre of Excellence
In addition to the largest worldwide capacity of commercial SPPS peptide production up to batch sizes exceeding 400 kg (> 35 Amino Acids), and a yearly capacity of > 2 Metric Tons of Peptide, CordenPharma's multi-pronged approach to becoming the #1 peptide CDMO supplier extends to small-scale capabilities,... -
Product Early-Phase Oral Solid Dose Drug Product Innovation Centre of Excellence
CordenPharma’s Drug Product Innovation Centre of Excellence offers early phase formulation development and manufacturing services for Oral Solid Dose (OSD) drug products, including Bioavailability Enhancement technologies. In collaboration with our Solid State Centre of Excellence, we provide integrated AP... -
Product Early-Phase Peptide Manufacturing Centre of Excellence
In addition to enhanced large-scale peptide manufacturing with the world's largest SPPS facility available, CordenPharma's multi-pronged approach to becoming the #1 peptide CDMO supplier extends to small-scale capabilities, which started with expansions of non-GMP peptide capacity back in Q4 2020. Now Cord... -
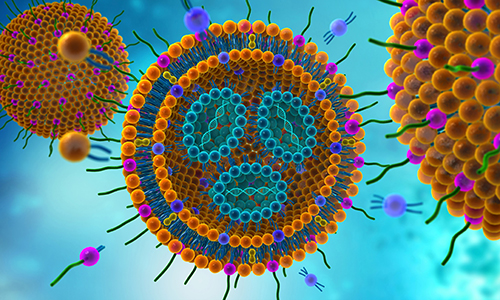
Product Lipid NanoParticle (LNP) Formulation
Delivering the capabilities pharma needs to support its drug strategies, CordenPharma offers advanced LNP formulation technologies and forward-integrated sterile injectable manufacturing services to create a proven commercialization pathway. We support requirements for Pre-filled Syringes (PFS), Vials, Am... -
Product CordenPharma Peptides Platform
Your Expert Partner for Peptide APIs
• >> Proven track record delivering world class manufacturing services from early stage to commercial supply, including complex products (e.g., Linaclotide, Enfuvirtide, Mifamurtide, Eptifibatide, Glatiramer, GLP analogues). • >> Proprietary... -
Product CordenPharma Oligonucleotide Platform
Your Expert Partner for Oligonucleotide APIs
• Process Development & Small-scale: scale-up to 10 mmol per run(small-scale equipment can also support early-stage supply)
• Scale-up & Small to Medium-scale: scale-up to 100 mmol per run(supported by OligoPilotTM equipment)
• ... -
Product CordenPharma Injectables Platform
Your Expert Partner for Clinical & Commercial Injectable Drug Product Supply
• >> Wide & balanced range of injectable services incl. capability for combining injectable drugs and devices (combination products) • >> Aseptic and Terminal Sterilization Fill & Finish Technologies f... -
Product CordenPharma Highly Potent & Oncology Platform
One Source for Highly Potent & Oncological Products
• Integrated Supply: APIs & Oral / Sterile Drug Products • State-of-the-art facilities able to handle API and Drug Product of the highest potency • Development, clinical trial and commercial manufacturing • Full-service offering includi... -
Product CordenPharma Small Molecules Platform
Full-Service Partner of Choice from Advanced Intermediates to Drug Products • Integrated network of cGMP facilities across Europe and US • 1,200 m3 of volumetric capacity in 20 L - 28,000 L reactors with various materials of construction • Clinical (Phase I-III) and ongoing commercial supply to multiple ... -
Product CordenPharma Peptides APIs
CordenPharma is one of the leading manufacturers of peptide Active Pharmaceutical Ingredients (APIs) with an unprecedented track record of experience in supporting our customer along the value chain ranging from early clinical to commercial in worldwide markets. Today we support more than 50 cGMP devel... -
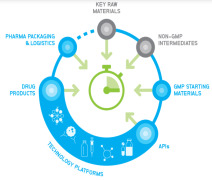
Product CordenPharma Fully-Integrated Supply
CordenPharma’s vertically-integrated supply chain model provides development & manufacturing expertise spanning the complete GMP supply chain. Beginning with regulated Key Raw Materials and Non-GMP Intermediates, our sister organization WeylChem supports back integration to sec... -
Product CordenPharma Sterile Liquid Drug Products
Sterile Drug Product manufacturing requires dedicated and specialized capabilities which are in compliance with FDA aseptic guidance and EMA regulatory requirements. CordenPharma has a long track record of experience in manufacturing Parenteral Sterile Solutions and Emulsions in different pharmaceutical fo...
Corden Pharma International GmbH resources (57)
-
News CordenPharma submits greenhouse gas emission reduction targets to SBTi
CordenPharma has submitted its near-term greenhouse gas (GHG) emission reduction targets to the Science Based Targets initiative (SBTi) for approval, marking a major step in its climate action commitments.
-
Video Webinar On Demand > Critical Aspects of a Robust Highly Potent Compound Program > The Importance of Understanding Intangible Elements of Containment
Register for this Live (4-4-18) or on Demand webinar to learn how the most important (and often over-looked) aspect to a robust containment program is the strength of the intangible, softer elements. Discuss how to maximize the success of your engineering control investments and allow for continued alignment to your company’s commitment to providing a workplace free of recognized health & safety hazards. -
News CordenPharma signs billion dollar agreement to manufacture new peptide
CordenPharma, the CDMO specialising in APIs, lipid excipients, and drug products, signs a new billion dollar contract to expand its manufacturing capabilities. -
Video Webinar On Demand > From Market Compliance to Business Supply: The Necessity for Serialization
This recorded webinar archive addresses the necessity of serialization from market compliance to business supply. Expert speaker Mario Scigliano covers all aspects of serialization, from line choice and regulation approach to management of data and related products.
-
News CordenPharma joins sustainability initiative to achieve Net Zero
The CDMO has joined the environmental initiative looking to align private sector companies with those aims upheld by the Paris Agreement to reduce global warming. -
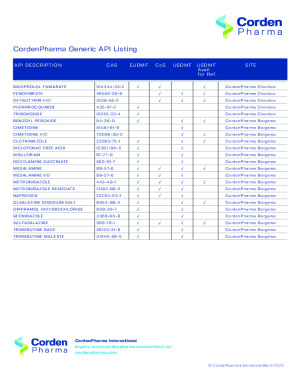
Brochure Generic API List
For generic drug companies, CordenPharma supplies more than 50 Active Pharmaceutical Ingredients (APIs), including controlled substances, for pain management, cardiovascular, gastrointestinal, central nervous system, anti-infective and respiratory indications. We manufacture these products using complex and technical expertise while maintaining a long-standing reputation of quality and reliability.
-
News CPHI Frankfurt 2022: Innovator Interview – CordenPharma
In this interview from CPHI Frankfurt 2022, we speak to Dr Michael Quirmbach of CordenPharma about his role as President and CEO and what CordenPharma is bringing to the pharma industry. -
Brochure Catalog Lipid List > Readily Available Phospholipids
CordenPharma offers a wide selection of Readily Available Derivatized Phospholipids suitable for Research & Discovery for purchase in convenient 1 g and 10 g pack sizes. -
News Press Release > Astorg to Acquire Leading Pharmaceutical Contract Development & Manufacturing Organization CordenPharma from ICIG
2 May 2022 – Basel >
Astorg and International Chemical Investors Group (“ICIG”) today announce that they have signed a binding agreement whereby Astorg will acquire CordenPharma (“CordenPharma” or the “Company”), a global leading pharmaceutical CDMO (Contract Development & Manufacturing Organisation) with highly differentiated capabilities in Active Pharmaceutical Ingredients, Excipients, and Drug Products, from ICIG.
-
Webinar LIVE WEBINAR > Your Injectable Roadmap: Tues Oct 13, 16:00 CEST / 10 EDT
Speakers: Mark Chipperfield (Corvus Ltd), Fabio Stevanon (CordenPharma), Umberto Romeo (CordenPharma)
Key Learning Objectives:
Review the fundamentals of combined injectable delivery device - drug products. Get an update on the latest regulation landscape for combined drug-device products, including some technical challenges for injectable Pre-Filled Syringes (PFS) & Auto-injectors. Gain insight into sound Injectable Drug Product strategy from a Full-Service CDMO industry leader. Learn about recent injectable industry changes facing Pharma, especially in the “New Normal” COVID-19 era, and how CordenPharma’s successful response & expanded capabilities can best support your drug development goals. Learn from an R&D Expert on ways to efficiently develop & commercialize an injectable drug. -
News Press Release > CordenPharma Completes Acquisition of Three Manufacturing Facilities from Vifor Pharma
1 February 2022 – Luxembourg > CordenPharma, a leading, full-service Contract Development & Manufacturing Organization (CDMO) supplying APIs, Excipients, Drug Products, and associated Packaging services, announced today the completion of the acquisition of three manufacturing facilities from Vifor Pharma, to be ultimately renamed Corden Pharma Fribourg S.A. (including its Ettingen branch) in Switzerland, and Corden Pharma Lisbon S.A. in Portugal.
-
Video Join CordenPharma’s Live Injectable Webinar: Tuesday, October 13th at 16:00 CEST / 10 EDT
Your Injectable Roadmap for Combined Drug Product - Delivery Device Regulations & Evolving Capabilities in the New Normal > How a Full-Service CDMO Supports Pharma -
News Press Release > CordenPharma Colorado Expands Lipid Excipients Purification
Luxembourg, 19 April 2021 -- In response to the COVID-19 pandemic, mRNA (messenger RNA) vaccines have catapulted to center stage of the pharmaceutical and biotechnology industry. As of early 2021, there are eight ongoing human trials for mRNA vaccines led by Moderna, BioNTech / Pfizer, CureVac, Sanofi / TranslateBio, Arcturus / Duke-NUS Medical School (Singapore), Imperial College London, Chulalongkorn University (Thailand), and Providence Therapeutics. -
Brochure Brochure > Weylchem InnoTec
WeylChem InnoTec are your innovative partner to the fine chemical, pharmaceutical and electronic industry. We provide world-class analytical, modern developmental and tailor-made manufacturing services. With our competencies in Analytics, Contract Development & Custom Synthesis, WeylChem InnoTec is your agile development partner.
Our Services:AnalyticsContract DevelopmentCustom SynthesisAdvanced technologies
How We Support You in Different Markets:
Organic ElectronicsCatalysts and LigandsSustainable ChemistryPharmaceutical IntermediatesAgrochemicalsVisit weylchem-innotec.com or email InnoTec@weylchem.com for more information. -
News Top 5 Industry Content Reads on CPHI Online This Month
If you’re looking for news, product information and market trends from leading pharma companies, the CPHI-Online.com Company Showcases are a great resource for buyers who want to stay up to date, browse product portfolios and find the right partner.
-
Video CordenPharma ONE PARTNER Video
As your ONE PARTNER, CordenPharma provides you with an integrated solution spanning all product life cycle stages, from preclinical to commercial, supported by dedicated regulatory and project management services. -
News Press Release > Pandemic Preparedness: WACKER & CordenPharma Will Produce mRNA Vaccines for Germany When Needed
11 April 2022 - Munich / Halle / Plankstadt- WACKER AND CORDENPHARMA TO BECOME PART OF GERMANY’S PANDEMIC PREPAREDNESS PLANTHE TWO COMPANIES WILL BE READY TO PRODUCE MRNA VACCINES FOR THE GERMAN GOVERNMENT IF THE COVID-19 PANDEMIC CONTINUES OR A NEW PANDEMIC OCCURS
- WACKER AND CORDENPHARMA JOINTLY COVER THE ENTIRE MANUFACTURING CHAIN FOR VACCINES
- HALLE WILL BECOME A COMPETENCE CENTER FOR PRODUCING MRNA VACCINES, WITH ADDITIONAL CAPACITY FOR OTHER CUSTOMERS AS WELL
- CORDENPHARMA WILL USE ITS YEARS OF EXPERIENCE IN PRODUCING CUSTOM AND STANDARD LIPIDS AND IN FILL & FINISH
- WACKER INTENDS TO INVEST OVER €80 MILLION ANNUALLY IN EXPANDING THE GROWTH OF ITS BIOTECHNOLOGY BUSINESS OVER THE NEXT FEW YEARS
-
Brochure CordenPharma Invests €900m in Transformational Peptide Platform Expansion
CordenPharma is making a record investment of ~€900m over the next 3 years in expanding its peptide platform, both at its Colorado, US site and in Europe. -
News Press Release > CordenPharma & Moderna Extend Lipid Supply Agreement for Moderna’s (mRNA-1273) Vaccine Candidate
Luxembourg, 28 May 2020 -- CordenPharma, a full-service Contract Development & Manufacturing Organization (CDMO) for the supply of APIs, Drug Products & Pharmaceutical Packaging, announces the signing of an amendment to their existing manufacturing agreement with Moderna, Inc. (Nasdaq: MRNA), a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients. -
Brochure CordenPharma API Crystallization Solid-State Brochure
We offer sophisticated process development addressing critical process parameters & quality attributes to deliver the most suitable crystalline form of an API. -
Brochure CordenPharma Lipids & Carbohydrates Platform Brochure
Custom & standard lipids manufacturing, including lipids for mRNA-based therapeutics, Lipid NanoParticle Starter kits & integrated Formulation / Fill & Finish. -
Brochure Subscribe to CordenPharma's Corden Connect Newsletter
Receive quarterly updates on CordenPharma's latest strategic investments & initiatives across all 6 technology platforms, as well as industry news & trends. -
Brochure CordenPharma Sets Science-Based Emission Reduction Targets for Sustainability
2023 marked the start of our campaign to evaluate & develop science-based targets for reducing greenhouse gas emissions in line with the SBTi initiative. -
Brochure PR 30 Mar 2023: Wacker, CordenPharma, LMU & HU Berlin use AI for RNA actives.
Project to accelerate use of AI algorithm to develop new generation of Lipid NanoParticles (LNPs), as key components of RNA-based drugs, with best formulations. -
Brochure Press Release 26 July '23:CordenPharma Forms Technology & Science Advisory Board
CordenPharma's new TSAB includes 8 world-class experts from academia & industry, who will provide strategic & scientific expertise to drive continued growth. -
Brochure Press Release 2 May 2023: CordenPharma Announces Entry into Oligo Manufacturing
CordenPharma enters the synthetic oligonucleotide manufacturing arena with capex investment in two phases and fully-integrated APIs to Drug Product offerings. -
Brochure Press Release 6 Sept 2023: CordenPharma Inaugurates Increased Peptide Production
CordenPharma inaugurates increased commercial peptide production with newly-upgraded facilities in Colorado, the largest SPPS manufacturing capacity worldwide. -
Brochure CordenPharma Drug Product Services
Drug product development & manufacturing for OSD and other non-sterile specialty dosage forms, and Aseptic / Terminal sterile dosage forms for all stages. -
Brochure CordenPharma Packaging Brochure
CordenPharma is an expert in contract manufacturing & packaging of various dosage forms with primary/secondary technologies from early-stage to commercial. -
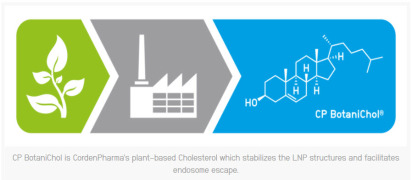
Brochure CordenPharma's Standard Lipid Catalog List
The lipid catalog offers multiple pack sizes of readily available derivatized, peg, phospholipids, ionisable, cationics & BotaniChol (cholesterol from plants). -
Brochure CordenPharma's Early-Phase OSD Drug Product Innovation Brochure
CordenPharma’s early-phase Drug Product services & bioavailability enhancement address formulations containing solubility- or permeability-challenged APIs. -
Brochure CordenPharma Small Molecules Platform Brochure
With over 12,000 L of capacity and reactors up to 28,000 L, we manufacture your API & 100s of millions of tablets/capsules with various formulation options. -
Brochure CordenPharma Highly Potent & Oncology Brochure
The Highly Potent & Oncology platform offers potent compounds with API scale-up from lab to 12,000 L vessels and OSD manufacturing starting from 100 g blend. -
Brochure CordenPharma Oligonucleotides Brochure
The Oligonucleotide platform supplies you with specialized combined expertise from early stage to commercial supply of advanced Oligonucleotide APIs. -
Brochure CordenPharma Injectables Platform Brochure
Our injectable services start from early formulation development to full commercial scale, covering both terminal sterilization & aseptic filling, including PFS -
Brochure CordenPharma Peptides Platform Brochure
The Backbone of Synthesis – Advanced Peptide APIs > Our scientists have mastered the synthesis of peptide APIs from early phase development to large-scale. -
Video Video - CordenPharma ONE PARTNER CDMO
Video highlighting CordenPharma as your ONE PARTNER CDMO for the fully-integrated supply of APIs, Excipients, Drug Products and Packaging across 5 Technology Platforms:InjectablesHighly Potent & OncologySmall MoleculesPeptidesLipids & Carbohydrates -
Video 15 Year Anniversary Facility Video Success Series
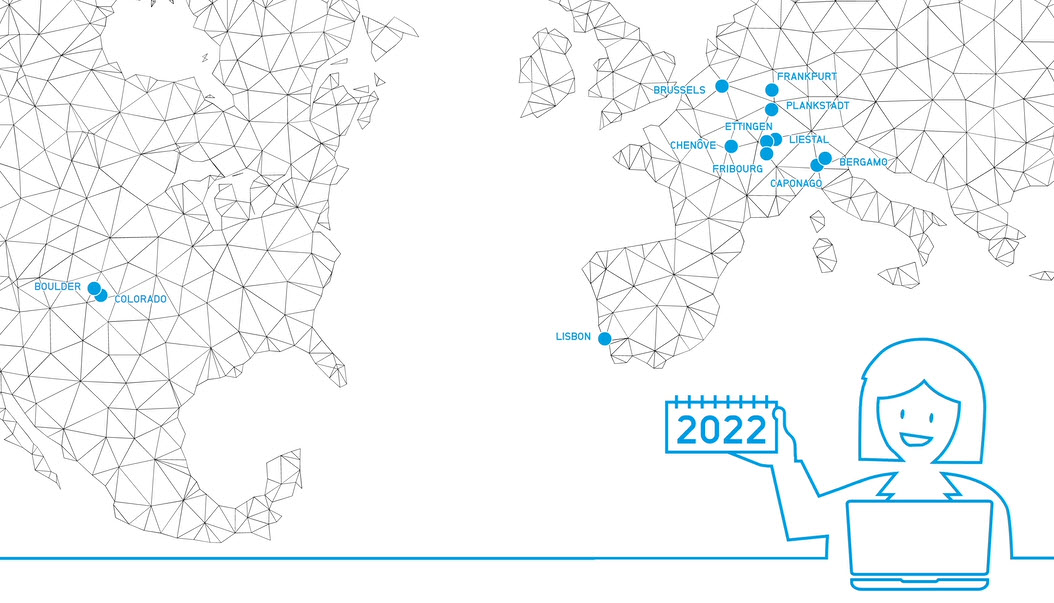
Since 2021 marks the 15 Year Anniversary of CordenPharma, we take a look back to our founding in 2006 with videos highlighting each of our API & Drug Product facility acquisitions that together, formed the foundation of our integrated supply network across Europe & the US. Thus began the journey of transforming former Large Pharma & Biotech assets into a ONE PARTNER CDMO. -
Brochure CordenPharma Switzerland Facility Fact Sheet
Small Molecule and Lipid Excipient Manufacturing in Liestal, CH -
Brochure CordenPharma Plankstadt Facility Fact Sheet
Highly Potent Drug Product Manufacturing in Plankstadt, DE -
Brochure CordenPharma Frankfurt Peptide R&D Fact Sheet
Peptide R&D Non-GMP Manufacturing in Frankfurt, DE -
Brochure CordenPharma Colorado Facility Fact Sheet
Peptide, Lipid Excipient and Highly Potent API Manufacturing in Boulder, CO, USA -
Brochure CordenPharma Chenove Facility Fact Sheet
API and Lipid Excipient Manufacturing in Chenove, FR. -
Brochure CordenPharma Caponago Facility Fact Sheet
Sterile Injectable Drug Product Manufacturing in Caponago, IT
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

































.jpg)