COMPLIANCE

Product Description
PHASE Compliance & Engineering Services.

-
IT
-
2020On CPHI since
-
25 - 49Employees
Company types
Categories
PHASE Compliance & Engineering Services.

-
IT
-
2020On CPHI since
-
25 - 49Employees
Company types
More Products from PHASE Compliance & Engineering Services. (1)
-
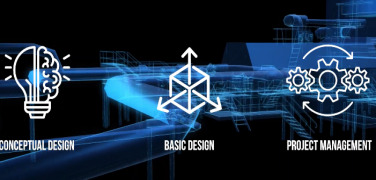
Product ENGINEERING
Conceptual study
Develop of Conceptual Study as first step of a project approach. This phase helps the user orthe investor to understand the technical feasibility of the project and to have a first economic impact defining a budget price.
Basic designBasic design development that ...
PHASE Compliance & Engineering Services. resources (1)
-
Brochure PHA.SE. Compliance, Validation & Engineering Services
PHA.SE. is a Milan-based company -set up in the year 2000- that prides itself on its expertise and on a dynamic and flexible approach. Over the years the company has consolidated its experience and extended its business to include a broad range of engineering, validation and quality assurance services. PHA.SE. delivers engineering, commissioning and validation services in accordance with standard, internationally recognized methodologies as well as cGMP regulation, European and US Pharmacopoeia.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






































-comp267516.png)








-comp323974.png)