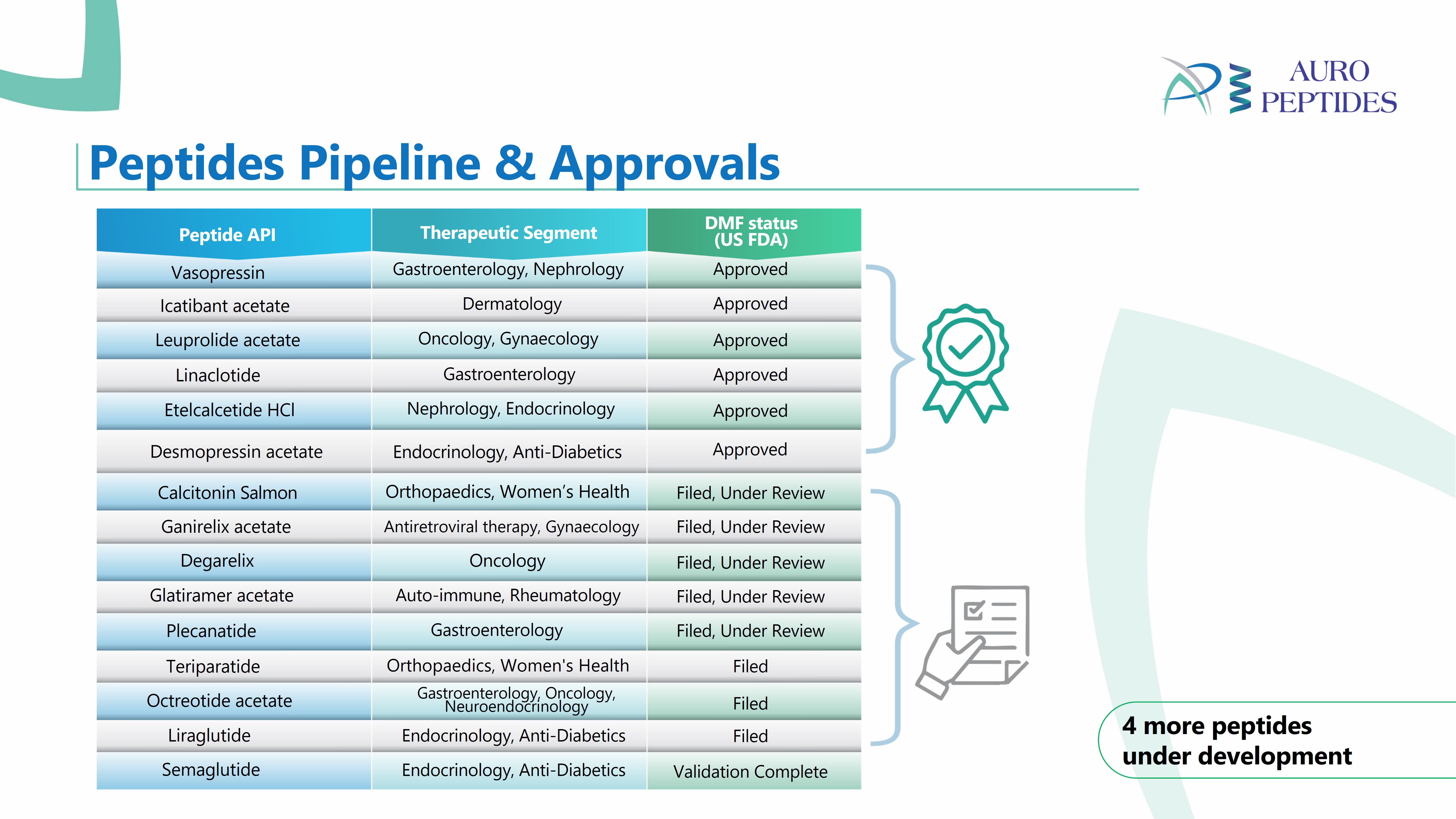
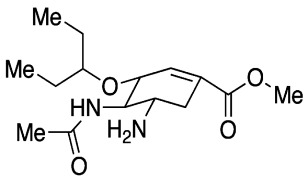
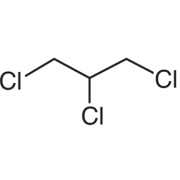
Colchicine Ph. Eur.

Product Description
Indena S.p.A.

-
IT
-
2015On CPHI since
-
5Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Indena S.p.A.

-
IT
-
2015On CPHI since
-
5Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Indena S.p.A. (41)
-
Product CBD
Natural, from Cannabis sativa L. -
Product COLCHICINE
Antigout -
Product COLCHICOSIDE
Chemical intermediate -
Product Thiocolchicoside B
Indena SpA offers a wide range of pharmaceutical products which includes thiocolchicoside b. Botanical origin: gloriosa superba l. seed. Biological activity: muscle relaxant. Contact us for more information. -
Product Valeroselect®
Indena SpA offers a wide range of pharmaceutical products which includes valeroselect®. Botanical origin: valeriana officinalis l. rhizome and root. Biological activity: sedative. Contact us for more information. -
Product Cannabidiol (from hemp)
Natural cannabinoid from hemp -
Product Colchicine Usp
Colchicine usp. Botanical origin: gloriosa superba l. seed. Biological activity: antigout. -
Product Docetaxel
Indena SpA offers a wide range of pharmaceutical products which includes docetaxel. Botanical origin: taxus baccata l. twig and leaf. Biological activity: anti-mitotic, anticancer. -
Product Paclitaxel
Paclitaxel belongs to the group of taxanes together with Docetaxel. It is marked by a powerful anticancer activity. This natural product, found as part of a National Cancer Institute program in 1962 aiming at identifying natural active compounds to potentially treat cancer, is a cell division inhibitor... -
Product Escin Extract
Indena SpA offers a wide range of pharmaceutical products which includes escin extract. Botanical origin: aesculus hippocastanum l. seed. Biological activity: antioedema. Contact us for more information. -
Product Escin Free Acid
Indena SpA offers a wide range of pharmaceutical products which includes escin free acid. Botanical origin: aesculus hippocastanum l. seed. Biological activity: antioedema. Contact us for more information. -
Product Escin Water Soluble
Indena SpA offers a wide range of pharmaceutical products which includes escin water soluble. Botanical origin: aesculus hippocastanum l. seed. Biological activity: antioedema. Contact us for more information.
Indena S.p.A. resources (15)
-
Video From the Rainforest to the Clinic. Turning a High-Potency Rare Natural Product Into an Anticancer API
Natural products can be characterized by complex structure, limited availability, and exceptional potency, whose combination makes it challenging their development as API. Success requires integration of expertise in different areas, including the construction of a sustainable supply chain for the starting biomass, the capacity to manipulate high-potency products in a GMP environment, and the versatility to strategically complement isolation with semi-synthesis, total synthesis and biotechnology. In this context, mutual thrust between the service provider company and the drug candidate owner is critical, and the successful development of the anticancer drug tigilanol tiglate will give the opportunity to address these issues. -
Whitepaper CDMO services
We have a unique expertise in developing and manufacturing HPAPIs. The company is equipped with kilolabs (for clinical and small commercial batches) and large industrial suite for HPAPIs with an OEL>20 ng/m3. -
Webinar Indena's Commitment to Sustainability: From Responsible Supply Chain Management to Smart Use of Energy, we aim to Protect Nature, People, and Business
Indena is relentlessly working to generate a sustainable impact on its stakeholders, cultivating with care its own precious circle: Nature, Technology, and People. In terms of actual projects and actions, Indena's commitment in sustainability includes responsible supply chain management, a circular economy in product design, and very smart use of energy in its plants. As for energy use, which is linked to climate change, one of the most urgent global sustainability challenges, Indena has been working for years to reduce consumption, save energy, and achieve high levels of energy self-production. All the actions taken for sustainability allow Indena to be fully reliable in terms of business continuity and thus be a solid partner for all its clients. -
Video CDMO: Health is a Joint Project
Indena’s view and technology expansion to support partners’ developments.
Supported by more than 100 year experience, Indena has been involved ever since in the CDMO activities on behalf of its partners, even before CDMO was so named. Starting from the extraction, chromatographic purification and isolation of natural derivatives, a core expertise of the company since its foundation, a significant technological expansion has been carried out over the years, thus now mastering fermentation (including toxic compounds), synthesis of potent and highly potent APIs and spray drying of difficult molecules.
Several technological solutions will be presented, highlighting cases where a combination of different techniques available have been instrumental to successful product development.
Did you enjoy this session? If so, you might like to visit our Connect to Frankfurt platform, where you can browse our collection of 30+ on-demand webinars and learn about our upcoming CPHI Frankfurt event, taking place 1-3 November in Frankfurt, Germany.
Bringing together the global supply chain under one roof, CPHI Frankfurt puts you at the heart of pharma. Can’t attend in person? You can access many of the event offerings online! Browse our exhibitor list, arrange meetings, view on-site content and network – all from home.
-
Podcast Podcast: HPAPI Manufacturing: Challenges, Solutions & Innovations
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. Growth in the highly potent active pharmaceutical ingredients segment means there is strong demand for specialist outsourcing solutions for their manufacture. CDMOs don’t just need to have the right equipment and facilities; they also need to convince sponsors that they possess the in-depth knowledge of safety practices and containment technology. In this CPHI Worldwide podcast with Carlo Aloni, Production and Process Technology Director at Indena, we looked at the various challenges of HPAPI production, the latest innovative technologies and what Indena is currently working towards in this highly promising growth sector. -
Podcast Podcast - CDMO Capabilities in Challenging Times
This podcast was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. When a pharmaceutical sponsor company decides it wishes to outsource vital functions such as manufacturing, drug development, and formulation, they are looking for several key qualities in a service provider. CDMOs need to provide flexibility, collaboration and innovation, as well as the ability to continuously evolve their offering. In this CPHI Worldwide podcast with Stefano Togni, Business Development Director at Indena, we explore how CDMOs are adapting to changing market conditions amid these challenging times. -
Podcast Podcast: Resilience and Sustainability – 100 years of Indena and Reflections on 2021
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. 2021 has been the second year of a very difficult and challenging global pandemic, to which the pharmaceutical industry has had to respond with resilience and flexibility. Coincidentally, it is also the centenary year of API contract developer and supplier, Indena. In this CPHI Worldwide podcast with Francesca de Rensis, Marketing Director at Indena, we take a look back at the proud 100 years of history that have shaped the company into the international business it is today as well as examining the main supply chain challenges facing that have defined the year 2021. -
Video Cannabinoids Status Quo
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. The session explores: Exploring the Medical Cannabis Opportunity for Pharma CBD as an API: What lies ahead Cannabis product development and design strategies Overview of the international cannabis product landscape Key considerations when incorporating pharmaceutical technologies Current and future areas of exploration Case Study: Medicinal Cannabis in The Netherlands - Status Quo -
Video Indena and its offering at a glance
In this video Stefano Togni, BD Director-CDMO, will highlight Indena latest technologies and offering in the CDMO area. -
Whitepaper Spray Drying services
Indena offers SD services from organic solvents (also in the presence of excipients). Equipped with two industrial SDs, the company is further investing in a smaller equipment dedicated to HIPOs. -
Whitepaper Indena CDMO technology overview
This presentation covers the full Indena technological offer for CDMO services. -
Whitepaper INDENA: CAPITALIZING ON HIGH CONTAINMENT EXPERTISE
This whitepaper gives an overview on the HPAPIs market, the technological challenges they pose and the approach Indena has adopted. -
Video Indena CPHI video interview 2019 - new implemented technologies
In this video, Indena's representative highlights the latest technological implementation and the ongoing technological expansion. It is covering the area of HPAPIs, Spray Drying from organic solvents, pilot pharmaceutical production of APIs and fermentation of toxic compounds. -
Video Indena at CPHI Worldwide 2018
Mr. Stefano Togni, BD & Licensing Director, Indena S.p.A. speaks with CPHI TV at CPHI Worldwide in Madrid
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













-file151652.png)


-file131247.png)