Clinical trial material (CTM) manufacturing and release

Product Description
Skyepharma Production S.A.S

-
FR
-
2017On CPHI since
Categories
Skyepharma Production S.A.S

-
FR
-
2017On CPHI since
More Products from Skyepharma Production S.A.S (15)
-
Product Soctec® Technology_Self Orienting Capsule Technology
Soctec® was developed for drugs that need to be retained in the stomach for an extended period of time to then being slowly released either for a local effect in the stomach or for absorption in the upper intestine. Soctec® is a versatile extended release gastro-retentive platform technology that ... -
Product Medicated chewing gum technology
Our proprietary medicated chewing gum technology uses an approach similar to multilayer tableting. A combination of layers modulates the release rate of the active ingredients and flavouring agents when the gum is chewed. -
Product Microfluidizer technology
By using microfluidization technology process, Skyepharma provides solution to increase bioavailabity of poorly soluble APIs (micro and nanoparticule). It is also an interesting technology for micro and nano emulsion manufacturing. -
Product Regulatory services
NDA/ANDA MAA-CTD writing Regulatory support -
Product Worldwide production and supply (US, EU, Russia, Asia/Pacific, EMEA, Latam)
Certifications : FDA, Europe, ANVISA, Korean FDA etc. -
Product Early stage formulation Development & Process Design
We have extensive experience in solving complex formulation challenges. Our experts can apply our extensive range of proprietary technologies for immediate (IR) and modified (MR) / sustained, slow (SR) / controlled (CR) / extended (XR, ER) / pulsed / timed release as well as enteric and gastro reten... -
Product Manufacturing of complex and classic oral solid dosage forms
Manufacturing of classic and complex oral solid dosage forms: single layer tablets, multi-layer tablets, tab-in-tab, tab-in-caps, hard gel capsules, powder & pellets. -
Product Analytical Development and expertise
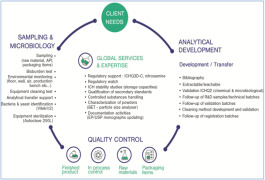
Our analytical experienced team support the pharmaceutical development during every phase of the development process. Analytical development transfer and validation, characterization capabilities, stability analysis, ICH Q3D (residuals solvent risk assesment), ICHQ3C (elemental impurities risk assesment... -
Product Scale-up and Quality by Design (QbD) processes
Skyepharma is experienced with the scale-up of complex oral solid dosage forms from laboratory to pilot-scale and full commercial scale manufacturing. We have powerfull compaction simulation tools ( STYL'One evolution) allowing to derisk and fasten scale-up steps.
Our capabilities :... -
Product Packaging in Blister
Primary packaging: Forming station for both PVCs and aluminium and all kind of feeder for tablets and capsules. Secondary packaging: Labelling, carton and casepacker. -
Product Packaging in Bottle
Primary packaging : Plastic and glass bottles (round and square) loading station. Secondary packaging : sealing, labelling, carton and casepacker. -
Product Packaging in Stick Pack
We can offer batch sizes from a few kilograms to several hundreds and the equipment is versatile in terms of material: powder, granules or pellets can be filled with PET / ALU / PE complex films up to 17 mm wide and up to 150 mm long.
Skyepharma Production S.A.S resources (24)
-
News Skyepharma selects Veratrak platform to ensure secure data storing and sharing
Using Veratrak's platform, the CDMO will have full oversight of data, including an audit trail of data and document processes -
Whitepaper Recent development in complex tablet formulation design
In this article, Mathieu Degomme, PharmD, Pharmaceutical Development Project Manager, and Aline Moulin, PhD, Pharmaceutical Development Director, both of Skyepharma, outline three case studies from recent early-stage development programmes to illustrate what kind of complex tablets can be developed to meet specific target dissolution profiles for versatile applications. -
News R&D: Collaborative thesis on the mechanical resistance of tab-in-tab tablets - Progress to date.
R&D : Mechanical resistance of pharmaceutical press coated tablets (tab-in-tab): characterization and link with material and process parameters. -
Brochure Company brochure
Through 25 years of involvement and achievements in the pharmaceutical industry, Skyepharma’s development and manufacturing facility is a leader in the field of classic and complex oral solid dosage forms. -
News Formulation development : Ongoing orphan drugs development projects
Several ongoing development programs are dedicated to the manufacturing of orphan drugs intended for both US and European patients. Skyepharma team is proud to work on orphan drugs development projects. -
Brochure Skyepharma booklet of services
We support our clients through the whole product value chain with expert teams involved at the very beginning of every project. - Pharmaceutical development services - Technology offer for complex oral solid forms - Analytical services - Manufacturing & packaging capabilities - Serialization & aggregation service ready -
News Supply Chain Reliability at Skyepharma, David Lescuyer's Interview
This article describes the robustness of Skyepharma's supply chain. The delivery of our products has not been impacted during this COVID-19 pandemic. Discover how! -
News Skyepharma overcomes Russian serialisation complexities
Skyepharma has extended its serialisation and aggregation service to ‘hard to reach’ countries like Russia and Brazil. We are now able to offer serialized and aggregated supplies to Russia. -
News Skyepharma Analytical Services : Specialized teams
Skyepharma is a full CDMO. We have an analytical service that covers all aspects from development to manufacturing. Packaging, raw materials and finished products are all controlled by this service. -
Whitepaper CPHI 2018_Pharma Insight Briefings
Aline Moulin, Head of New Product Introduction Department (NPI), presented different case studies during the Pharma Insight Briefings 2018 (CPHI Worldiwide): "COMPACTION SIMULATION AS A POWERFUL QUALITY BY DESIGN (QbD) TOOL How Skyepharma can help you to shorten the time and reduce the cost of pharmaceutical development programmes" -
News Serialization and aggregation in the pharmaceutical industry
The serialization system allows the verification of a drug's compliance from the distribution to the delivery then to the patient. This article explains the serialization and aggregation implemented at Skyepharma. -
Video Skyepharma at CPHI Worldwide 2018
David Lescuyer, Executive Vice President and Frederic Checo, Pharmaceutical Development Leader, both from Skyepharma speaks with CPHI TV at CPHI Worldwide in Madrid, Spain -
News Skyepharma set-up for serialization and aggregation, ready for Russian market
Since 2018, oral dosage CDMO Skyepharma radically updated and expanded its packaging capabilities, opening new packaging lines equipped for serialization and aggregation. -
Whitepaper CPHI 2017_Pharma Insight Briefings
Lucile Kowalsky, Project Manager in the New Product Introduction (NPI) department presented a case study during the Pharma Insight Briefings (CPHI Worldwide 2017) : "Quality by Design and Expertise:Accelerating time to market of complex oral solid dosage Accelerating time to market." -
Video Skyepharma - Packaging services 2020
- Bottle packaging - Blister packaging
- Stick-packs
- Serialization & aggregation services (Seavision systems) -
Video Skyepharma - "What do we mean by Fully Integrated CDMO ?"
Skyepharma is a french fully integrated CDMO with expertise in classic & complex oral solid dosage forms offering : Development, Manufacturing and Packaging services. As a fully integrated CDMO Skyepharma provides tailor-made solutions to customers by positioning itself as an integrated partner offering a wide range of premium services.
Our experienced team of specialists works to optimize target product profile and drug development program from early formulation development, scale-up, manufacturing and packaging to regulatory support. -
Video Skyepharma - "What do we mean by Agile CDMO ?"
For Skyepharma, to be agile qualifies our ability to mobilize experts and work together to solve complex efficiently and rapidly. Skyepharma, your AGILE CDMO partner for tailor-made solutions. -
Video Skyepharma - "What do we mean by Reliable CDMO ?"
For Skyepharma, being reliable means taht we are able to meet our customers' needs on time and to the expected level of quality. Skyepharma your reliable CDMO for faster time to market without compromising on product quality -
Whitepaper Analytical Development & Technico Regulatory Expertise
We are able to develop analytical methods in a limited time, from bibliography study, screening parameters and conditions to sample preparation and assessment of robustness. -
Video CPHI Worldwide 2019 | Interview with Skyepharma
.Skyepharma's interview shows the company's growth since last year. David Lescuyer, Florence Lemoult and Frédéric Checot are the interviewees
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











































































































.png)




