Cinitapride Hydrogen Tartrate
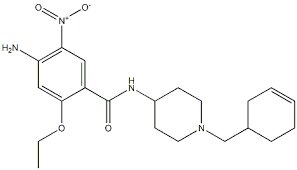
Product Description
Metrochem Api Pvt Ltd

-
IN
-
2015On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Metrochem Api Pvt Ltd

-
IN
-
2015On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Metrochem Api Pvt Ltd (66)
-
Product Antazoline Phosphate
Antihistamine - Commercial -
Product Azilsartan Medoxomil Potassium
Antihypertensives - Commercial -
Product Bempedoic Acid
Antihyperlipidemic - Under Development -
Product Bepotastine Besilate
Antihistamine - Commercial -
Product Bilastine
Antihistamine - Under Development -
Product Canagliflozin
Antidiabetic - Commercial -
Product Clopidogrel Bisulphate (form I & II)
Antiplatelet - Commercial -
Product Crisaborole
Atopic Dermatitis - Under Development -
Product Dabigatran Etexilate Mesylate
Antithrombotic / Anticoagulant - Commercial -
Product Dapagliflozin
Antidiabetic - Commercial -
Product Dexketoprofen Trometamol
Analgesic - Commercial -
Product Dexlansoprazole
Antiulceratives - Commercial
Metrochem Api Pvt Ltd resources (7)
-
News Metrochem was awarded India Pharma Bulk Drug Company of the Year 2020
Metrochem API Private Limited received the India Pharma- Bulk Drug Company of the year Award 2020 from the Government of India for outstanding contribution in the field of API Bulk Drugs. -
Brochure Metrochem API Private Limited API Brochure FY 24-25
Metrochem API Pvt ltd is one of the few vertically integrated Indian Pharmaceutical manufacturer approved by USFDA (EIR), WHO-GMP, COEFPRIS, KFDA & ISO 9001:2008 certification. We hold few process patents .Our products include API`s, Pellets and intermediates,CRAMS and P2P in wide variety of segments, mainly Anti Ulcerants, Anti allergic, Anti coagulants, Anti diabetic & Anti Hypertensive’s. Metrochem API Vizag`s manufacturing unit is a green field manufacturing facility in 50,000 sqmts with 700kl reaction volume. We have capability to take a kilo to multi ton batches in the new facility. This USFDA and EUGMP approvable facility started operations in May-2015 -
Video Corporate Video
Metrochem API Pvt ltd is one of the few vertically integrated Indian Pharmaceutical manufacturer approved by USFDA (EIR), WHO-GMP, COEFPRIS, KFDA & ISO 9001:2008 certification. We hold few process patents .Our products include API`s, Pellets and intermediates, CRAMS and P2P in wide variety of segments, mainly Anti Ulcerants, Anti allergic, Anti coagulants, Anti diabetic & Anti Hypertensive’s. Metrochem API Vizag`s manufacturing unit is a green field manufacturing facility in 50,000 sqmts with 700kl reaction volume. We have capability to take a kilo to multi ton batches in the new facility. This USFDA and EUGMP approvable facility started operations in May-2015 -
Brochure Metrochem Overview
Metrochem API Pvt ltd is one of the few vertically integrated Indian Pharmaceutical manufacturer approved by USFDA (EIR), WHO-GMP, COEFPRIS, KFDA & ISO 9001:2008 certification. We hold few process patents .Our products include API`s, Pellets and intermediates,CRAMS and P2P in wide variety of segments, mainly Anti Ulcerants, Anti allergic, Anti coagulants, Anti diabetic & Anti Hypertensive’s. Metrochem API Vizag`s manufacturing unit is a green field manufacturing facility in 50,000 sqmts with 700kl reaction volume. We have capability to take a kilo to multi ton batches in the new facility. This USFDA and EUGMP approvable facility started operations in May-2015 -
Brochure Metrochem API Private Limited Presentation
Metrochem API Pvt Ltd was founded by Dr. N.V. Rao (PhD in Organic Chemistry) in the year 2004, with a grand vision of being the “most preferred supply partner to pharmaceutical customers worldwide” through timely deliveries, quality products and stellar customer service. Over the last 15 years, Metrochem API Private Limited has evolved into one of the fastest growing manufacturers of Active Pharmaceutical Ingredients (APIs), Pellets (Semi finished Formulations) and Intermediates. Our strong commitment to providing quality products is boasted by in-depth industry knowledge, a well-qualified team of professionals, as well as hi-tech and advanced infrastructure. We have 6 manufacturing facilities in Hyderabad and Visakhapatnam, catering to our API, Pellet and Intermediate needs. We have a 2500 strong workforce manning these facilities. Our manufacturing facilities are approved by several international regulatory bodies such as ISO 9001-2015, USFDA, WHO: GMP, PMDA, COFEPRIS and KFDA. Our R&D center, sprawling across a 20,000 sqft campus in Hyderabad, is the backbone of our success. -
Brochure Intermediates Brochure FY 24-25
Metrochem API Pvt Ltd was founded by Dr. N.V. Rao (PhD in Organic Chemistry) in the year 2004, with a grand vision of being the “most preferred supply partner to pharmaceutical customers worldwide” through timely deliveries, quality products and stellar customer service. Over the last 15 years, Metrochem API Private Limited has evolved into one of the fastest growing manufacturers of Active Pharmaceutical Ingredients (APIs), Pellets (Semi finished Formulations) and Intermediates. Our strong commitment to providing quality products is boasted by in-depth industry knowledge, a well-qualified team of professionals, as well as hi-tech and advanced infrastructure. We have 6 manufacturing facilities in Hyderabad and Visakhapatnam, catering to our API, Pellet and Intermediate needs. We have a 2500 strong workforce manning these facilities. Our manufacturing facilities are approved by several international regulatory bodies such as ISO 9001-2015, USFDA, WHO: GMP, PMDA, COFEPRIS and K... -
Video Chemical Engineering in Pharmaceutical Manufacturing
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 This session will address: The role of engineering in the synthesis of organic compounds How to ensure smooth and effective scale-up from lab to plant? Controlling PSD through crystallization? Basic principles of solvent recovery systems. Process safety considerations Troubleshooting manufacturing processes
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

































-file117056.png)