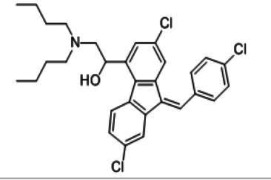
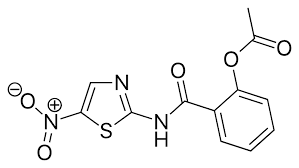
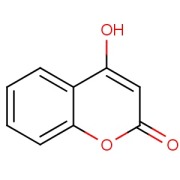
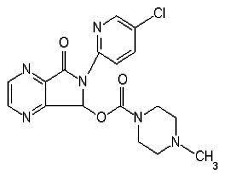
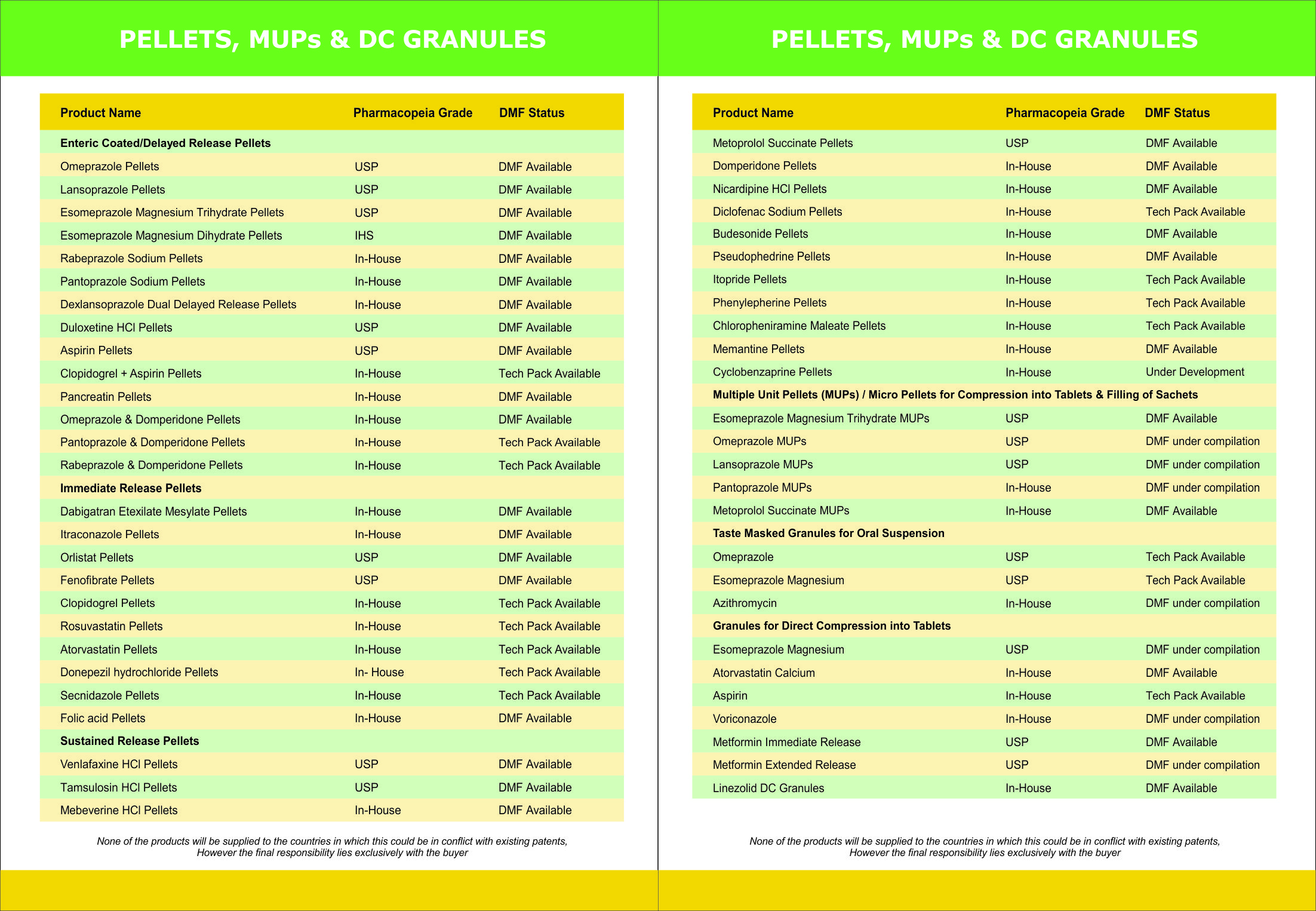
CILOSTAZOL

Product Description
Blanver Farmoquimica Ltda.

-
BR
-
2015On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Categories
Blanver Farmoquimica Ltda.

-
BR
-
2015On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
More Products from Blanver Farmoquimica Ltda. (7)
-
Product Tenofovir Disoproxil Fumarate 300 mg + Emtricitabine 200 mg
- Presentation: Plastic bottles with 30 coated tablets.
- Indication (Brazil Prescription Medicines):
1) For PrEP® (pre-exposure prophylaxis), it can help reduce the risk of getting HIV-1 through sex, when taken every day and used together with safer sex practices.
... -
Product Raloxifene Hydrochloride 60 mg
- Presentation: Blisters containing 28 film-coated tablets
- Indication (Brazil Prescription Medicines):
1) For the prevention and treatment of osteoporosis;
2) To reduce the risk of breast cancer in postmenopausal womem with osteoporosis.
-... -
Product Tenofovir Disoproxil Fumarate
API - Active Pharmaceutical Ingredient
- Therapeutic Area: HIV infection and Chronic Hepatitis B
- Status: DMF (Drug Master File Ready)
Blanver fully respects the patents’ expiration dates in all countries
-
Product Tenofovir Disoproxil Fumarate 300 mg
- Presentation: Package containing 60 plastic bottles with 30 coated tablets.
- Indication (Brazil Prescription Medicines): First line medication indicated, in combination with other antiretroviral agents, for the treatment of HIV-1 infection in adults and chronic Hepatitis B.
... -
Product Sofosbuvir 400 mg
- Presentation: Plastic bottles with 28 coated tablets.
- Indication (Brazil Prescription Medicines): indicated for the treatment of chronic hepatitis C Virus infection in adult patients, in combination with other antiviral agents.
- Recommended Dosage: 1 tablet once a day... -
Product Tenofovir Disoproxil Fumarate 300 mg + Lamivudine 300 mg
- Presentation: Plastic bottles with 30 coated tablets.
- Indication (Brazil Prescription Medicines): Used with other HIV-1 medicines to treat HIV-1 infection. Its use can promote greater adherence to the treatment and also reduce the cost and number of pills taken daily.
... -
Product Lithium Carbonate
API - Active Pharmaceutical Ingredient
- Therapeutic Area: Bipolar Disorder and Major Depressive Disorder as adjunctive
- Status: DMF (Drug Master File) ready
Blanver fully respects the patents’ expiration dates in all countries*
Blanver Farmoquimica Ltda. resources (2)
-
Brochure Blanver Brochure
Please check our brochure. Contact us for more information. +55 11 3199-8700 | contatobr@blanver.com.br
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





.jpg)

























































%20(1)%20(1)-comp246058.png)