Chinese Hamster Ovaries (CHO) and cell lines
Chinese Hamster Ovaries (CHO) and cell lines Companies (6)
Chinese Hamster Ovaries (CHO) and cell lines News
-
News Novartis and Viatris latest facing lawsuit over HeLa cell misuse
Global pharmaceutical companies Novartis and Viatris are the latest hit with a lawsuit claim pertaining to alleged misuse of the ‘HeLa’ cell line from the estate of woman whose cancerous tissue cells were taken without consent. -
News Abzena and BioXpress Therapeutics partner to provide global biosimilar support
The combined capabilities will offer flexibility and innovation from small-scale biosimilar development through to large-scale manufacturing -
News ProBioGen and LAVA Therapeutics sign cell line development agreement
The agreement marks the second cell line development collaboration between the two companies -
News ProBioGen selected for development and large-scale manufacturing services
The Contract Development and Manufacturing Organization (CDMO) and technology provider will use its CHO.RiGHT expression platform for high-titer cell line development.
Chinese Hamster Ovaries (CHO) and cell lines Products (23)
-
Product Incubator shaker
The ISF1-Z incubator shaker is suitable for applications in laboratories, research centers and production facilities. The ISF1-Z complies with GMP (good manufacturing practice) requirements and regulations. It offers a large shaking capacity with a small footprint and many features allowing straightforward...
-
Product Specialty Cell Culture Media, Cell Lines and Contract Services for Baculovirus Expression Vector Systems
Expression Systems, now an Advancion company, is dedicated to supplying and servicing the cell culture and bio-industrial markets with innovative cell culture media formulations, as well as cell lines, molecular tools, reagents, and contract services. The company specializes in the baculovirus expression v...
-
Product A CDMO PARTNER FOR LIFE
FUJIFILM Diosynth Biotechnologies is an industry-leading cGMP Contract Development and Manufacturing Organization (CDMO) supporting the biopharmaceutical industry in the development and production of biologics, vaccines and cell and gene therapies. Our focus is to combine technical leadership in process d...
-
Product CHO and HEK stable cell line development
Fast and efficient delivery of high-yielding stable CHO or HEK clones, with a productivity of up to 8 g/L within 8-12 weeks. Your benefit: Success assurance with the best cell line, right from the start. https://excellgene.com/ https://excellgene.com/offerings/cell-line-development/ https://excellgene.com/...
-
Product Cell line development services
Cell line development services, including molecular cloning, CHO cell line development and lead clone selection, bioprocess optimization, DSP and analytical characterization of the target molecule.

-
Product Cell Line Development
KBI offers a full suite of CLD across both mammalian and microbial expression systems. KBI can apply established mammalian recombinant protein expression systems to provide rapid and industry-leading cell line generation services using: CHO-M Selexis SUREtechnology, CHO-ZN, CHO-DG44, CHO-S, and CHO-K1 GS. ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product E. Coli Host Cell Proteins
ELISA methods are used to test for the presence of residual host cell proteins left in a drug or therapeutic protein following purification.
-
Product Bioprocess Development
Integrated upstream and downstream process development for high-yield manufacturing of biologics of desired quality. Your benefit: Cost-effective and scalable bioprocess with robust and reliable tech transfer. https://excellgene.com/offerings/downstream-process-development-dsp/ https://excellgene.com/offer...
-
Product cGMP Cell Bank Manufacturing
cGMP-compliant MCB/WCB preparation of in-house or brought-in CHO-/HEK-based cell lines. Your benefit: Risk-free and fast preparation, release of MCB/WCB. https://excellgene.com/offerings/cgmp-master-cell-banking/
-
Product Cell Host Technologies
Outlicensing of superior cell hosts: CHOExpress® & HEKExpress®, cell line generation with best in class transposon technology. Your benefit: In-house state of the art tech revolutionizing your development programs. https://excellgene.com/technologies/
-
Product Biosimilars
Portfolio of high-producing biosimilar cell lines and processes, off-the-shelf and generation of such from scratch. Biosimilarity optimisation of your recombinant cell line. Your benefit: Cost and time-savings, while bringing your product to the market. https://excellgene.com/offerings/biosimilars/
-
Product Media & Feeds for CHO cell line
The First CHOice® Medium is a high-performance cell culture medium optimized for mammalian suspension cells ensuring high product quality and cell viability during all cultivation phases. In combination with the First CHOice® Feeds Alpha & Beta, offer a platform to achieve excellent cell growth, an...
-
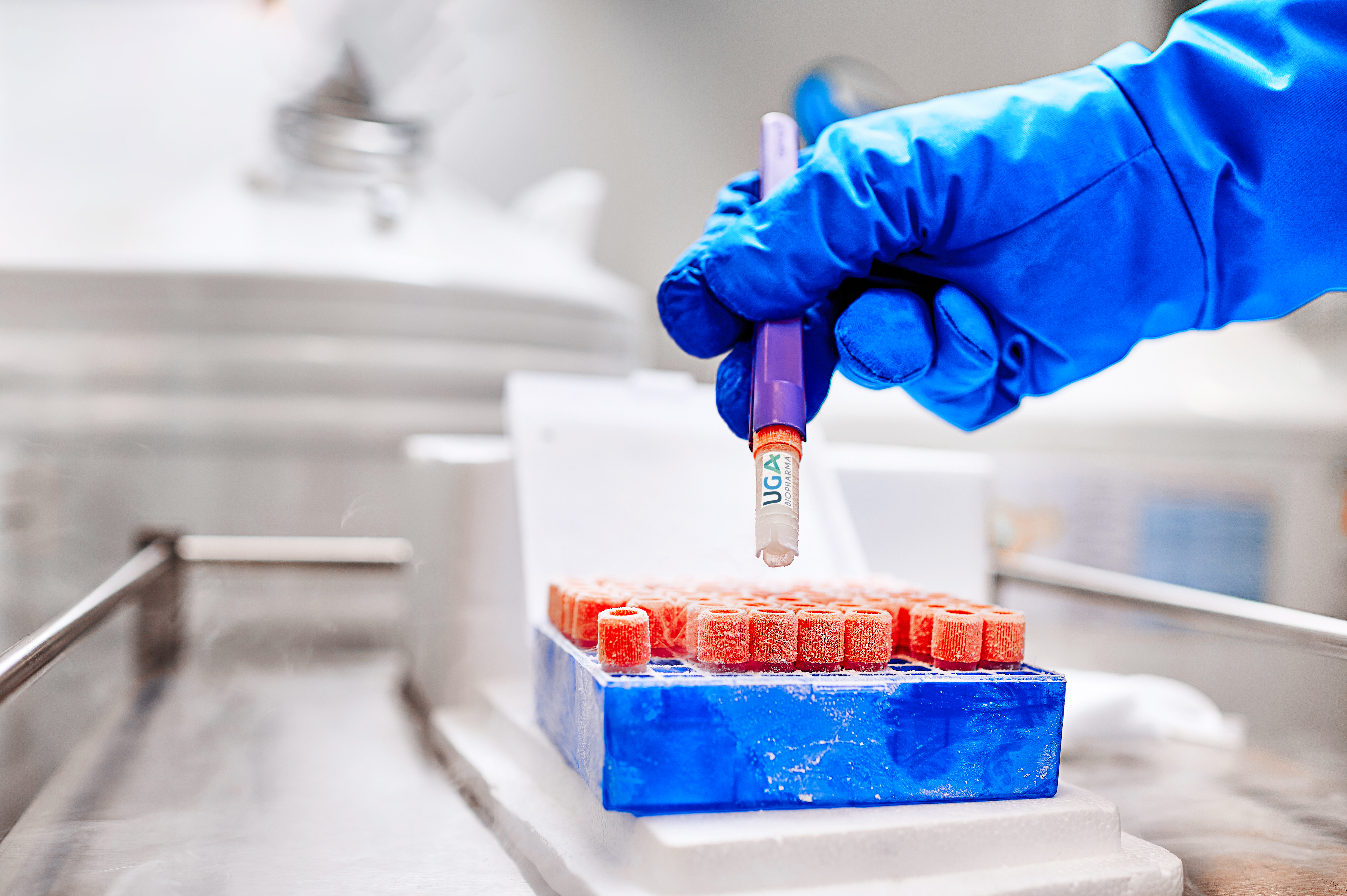
Product Ready-to Use CHO cell lines
UGA Biopharma GmbH offers to their clients so called Ready-to-use Biosimilar Cell Lines. This are in-house developed CHO cell lines stably expressing different biosimilar molecules, that are instantly available for in-licensing. The client profits, because the cell lines are directly available, and develop...
-
Product Chinese Hamster Ovary Host Cell Protein Testing
ELISA methods are used to test for the presence of residual host cell proteins left in a drug or therapeutic protein following purification.
-
Product LN₂ Generator
1. Product Introduction
1) Various sensor monitoring and alarm programs, two-way communication capabilities
2) Vacuum insulation piping for cryogenic
3) Self-generated liquid nitrogen in Cryogenic environments
4) One-touch company size
5) Low noise
6) Boil off g...
-
Product Cancer Cell Lines- research use only
Cancer cells are transformed cells that have acquired a series of changes that permit them to form tumors. They arise when the genes responsible for regulating cell division are damaged. The uncontrolled and often rapid proliferation of cells can lead to benign or malignant tumors. Beni...
-
Product GMP Cell & Virus Bank Storage
Our GMP-compliant storage area offers dedicated or shared storage in standard freezers (-80°C), ultra-low freezers (-130°C), or in cryotank for storage in liquid nitrogen vapor phase at temperature below -150°C.
-
Product Cell & Virus Bank Characterization
The safety testing of cell or virus banks at the level of the Master, Working and End of Production Cell Bank is a key element for cell- or virus-derived biopharmaceutical products. Characterization tests aim to confirm identity, genetic stability and purity of the cell or virus bank.
Upcoming Events
-
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






.png)



.jpg)




















.png)







.png)

.png)



.jpg)


