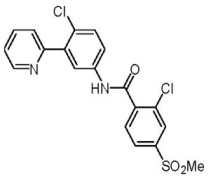
CGMP Psilocybin
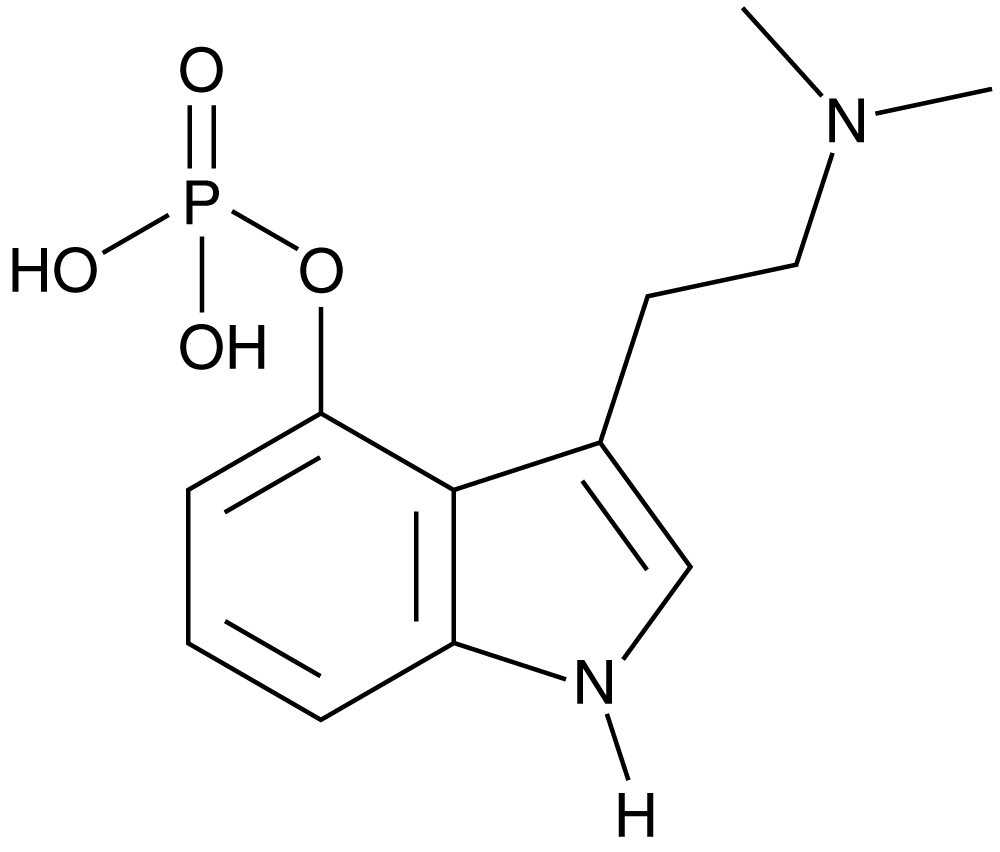
Product Description
Cayman Pharma s.r.o

-
CZ
-
2015On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
Cayman Pharma s.r.o

-
CZ
-
2015On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from Cayman Pharma s.r.o (7)
-
Product CGMP Bimatoprost
Bimatoprost is a potent FP receptor agonist that finds clinical use as an ocular hypotensive agent for the treatment of glaucoma. The DMF is on file with the US FDA, Brazil, Canada and India. -
Product CGMP Epoprostenol sodium salt
Epoprostenol is a potent vasodilator and antiplatelet substance with a very short physiological half-life. It is used for the treatment of pulmonary hypertension. The DMF for Epoprostenol Sodium Salt is on file with the US FDA, Canada, Japan, Australia, Switzerland and most EU member states. -
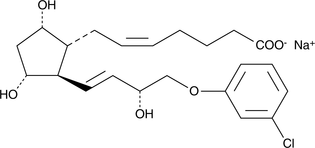
Product CGMP Cloprostenol sodium salt
Cloprostenol sodium salt is used in veterinary medicine as a luteolytic agent for the induction of estrus and the treatment of reproductive disorders in cattle, swine, and horses. Cloprostenol sodium salt is manufactured at Cayman Pharma both in racemic form (CAS No.: 55028-72-3) and optically active form ... -
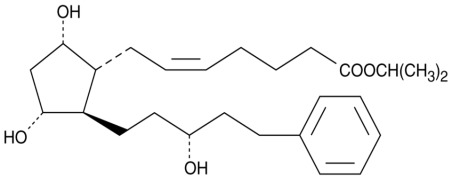
Product CGMP Latanoprost
Latanoprost is the most widely used prostaglandin for the treatment of elevated intraocular pressure in glaucoma. Latanoprost is manufactured at Cayman Chemical (Ann Arbor, MI, USA) and at Cayman Pharma (Czech Republic). GMP material from both Cayman Chemical and Cayman Pharma, which conforms to the USP, E... -
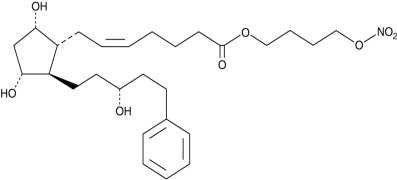
Product CGMP Latanoprostene Bunod
Latanoprostene Bunod is a nitric oxide-donating FP receptor agonist; it can be used for the treatment of elevated intraocular pressure (IOP) in glaucoma. Latanoprostene Bunod is manufactured at Cayman Pharma, our Czech subsidiary. GMP material is available, and the DMF for Latanoprostene Bunod is available... -
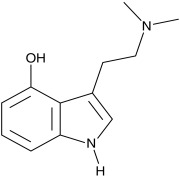
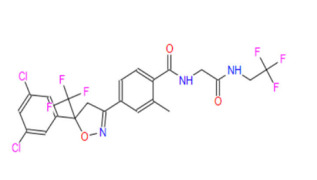
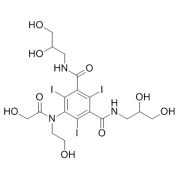
Product CGMP Psilocin
Psilocin is the active form of Psilocybin, a naturally occurring psychedelic prodrug produced by more than 200 species of fungi. Psilocin is manufactured at Cayman Chemical in Ann Arbor, MI via a fully synthetic route. GMP material appropriate for clinical trials is available. -
Product CGMP (+)-Cloprostenol (sodium salt)
(+)-Cloprostenol is the active enantiomer of Cloprostenol. Cloprostenol is used in veterinary medicine as a luteolytic agent for the induction of estrus and the treatment of reproductive disorders in cattle, swine, and horses. The ASMF for (+)-Cloprostenol (sodium salt) is on file in several EU member states.
Cayman Pharma s.r.o resources (13)
-
News Cayman Chemical Receives CEP for Latanoprost API from EDQM
ANN ARBOR, MI, SEPTEMBER 12, 2023—Cayman Chemical, a world leader in the manufacture of prostaglandins for both research use and as active pharmaceutical ingredients (APIs), has been granted a Certification of Suitability to the monographs of the European Pharmacopoeia (CEP) by the European Directorate for the Quality of Medicines and HealthCare (EDQM) for its latanoprost API, effective August 28th, 2023. Cayman Chemical is the first company in the USA to receive a CEP for latanoprost. -
Brochure Cayman Pharma cGMP API Manufacturing
This leaflet presents cGMP API portfolio of Cayman Pharma and Cayman Chemical. -
Brochure Cayman Chemical Contract Services
This leaflet presents services offered by Cayman Chemical. -
Brochure Cayman Pharma and Cayman Chemical - Opthalmics
This leaflet presents detailed overview of cGMP prostaglandins used for glaucoma treatment manufactured by Cayman Pharma and Cayman Chemical. -
Brochure Cayman Chemical - Psychedelics
This leaflet presents detailed overview of cGMP psychedelic APIs manufactured by Cayman Chemical. -
Brochure Cayman Pharma - Vascular APIs
This leaflet presents detailed overview of cGMP prostaglandins used for pulmonary hypertension treatment manufactured by Cayman Pharma -
Brochure Cayman Pharma - Veterinary APIs
This leaflet presents detailed overview of cGMP prostaglandins used in veterinary medicine and manufactured by Cayman Pharma -
Brochure API Manufacturing
Learn more about the importance of process understanding and Cayman's commercial API production -
Brochure API Contract Services
Cayman Chemical’s API Services group helps companies turn their new drug ideas into reality. The Cayman advantage offers the flexibility to support your project, allowing you to create a customized service to meet your needs. -
Brochure Ophthalmology APIs
The natural compound prostaglandin F2α (PGF2α) activates signaling pathways in the eye that reduce intraocular pressure (IOP). A family of PGF2α analogs, including bimatoprost, latanoprost, tafluprost, and travoprost, have been developed, and these compounds are more potent than PGF2α itself in lowering IOP and have fewer side effects. Moreover, they are known to be safe and effective when used in the treatment of glaucoma. -
Brochure Psychedelic APIs
Psychedelics are emerging as promising therapies in the treatment of various neurological and psychiatric conditions. Though psychedelics are controlled substances in most countries, clinical trials exploring the use of psychedelics are underway. The US FDA has granted breakthrough therapy status for the use of psilocybin in major depressive disorder and treatment-resistant depression, for which it has shown to be safe and effective. It may also have potential in the treatment of anxiety, substance use disorder, cluster headaches, as well as psychological distress associated with end-of-life. -
Brochure Vascular APIs
Each prostaglandin (PG) evokes distinct physiological effects through specific cell surface receptors. PGI2 (prostacyclin) promotes smooth muscle relaxation and inhibits platelet activation through the IP receptor. Agonists of IP, including epoprostenol, iloprost, and treprostinil, are effective in relaxing pulmonary arterial smooth muscle and are used in the treatment of pulmonary hypertension. -
Brochure Veterinary APIs
A stable and potent analog of prostaglandin F2α (PGF2α), cloprostenol is used in the care of domestic mammals to induce luteolysis, initiate estrus, and synchronize estrus cycles. Both the racemic and active isomer are also used to induce parturition, terminate abnormal and normal pregnancies, and treat chronic endometriosis and ovarian luteal cysts.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

































































































%20(1)%20(1)-comp246058.png)

