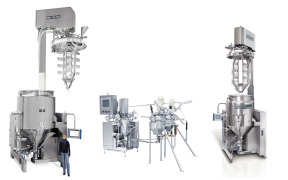
Centrifuge Bags

Product Description
Khosla Profil Pvt. Ltd.

-
IN
-
2019On CPHI since
Categories
Specifications
Khosla Profil Pvt. Ltd.

-
IN
-
2019On CPHI since
More Products from Khosla Profil Pvt. Ltd. (2)
-
Product Antistatic & Food Grade Fabric
We offer special Antistatic & Food Grade Fabric for meeting industrial safety needs. Our anti static fabrics are used in Fluid Bed Driers Bags, Centrifuge Bags etc.Manufactured with hi-tech Antistatic fiber/ yarn, our Antistatic Fabrics meet most stringen standards of quality to eliminate the stat... -
Product Antistatic Clean Room & ESD Lab Coat Fabrics for Pharmaceuticals
# Antistatic Fabric with 99% Polyester filament and imported conductive yarn# Effective eliminate static electric produced by human body; has the permanent antistatic function
# Advanced technology, clean cloths away debris, no dust
# Comfortable, wash resistant, wear resistant, good ventilation perf...
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











































![NexArni 50 [Valsartan with Sacubitril Tablets]](/46/product/129/06/57/picture.jpeg)