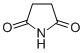
Leachables/Extractables/Particulates
Leachables/Extractables/Particulates Companies (4)
Leachables/Extractables/Particulates News
-
News EuTech announces new management team additions
EuTech has recently added two strong professionals in its Management Team. Shekhar Yerramilli has joined as COO and Rick Yglesias has joined as Head of Business Development. -
News Partnership formed to deliver effective biopharma industry testing
Stevanato and Pfeiffer Vacuum will provide biopharma companies with advanced container closure integrity analysis and testing protocols.
Leachables/Extractables/Particulates Products (13)
-
Product Nasal Drug Development
Development of nasal drugs: Scientists from our nasal drug development team provide method development, validation, and testing services to help you optimise the performance of your aqueous, powder, and propellant-driven nasal drug products. Conducted in Good Manufacturing Compliant (GMP) laborato...
-
Product Recipharm Analytical Solutions™
Through Recipharm Analytical Solutions™, we support customers with stand-alone analytical requirements. Our analytical development team has experience from developing hundreds of analytical methods every year, supporting development of formulations ranging from powder in capsules and IV solutions to ER tab...
-
Product Medicinal Cannabis
QPLAB has GMP Cannabis Testing licencing that allow to provide the following services: • Medicinal Cannabis Testing • Batch Testing • Enviromental Monitoring • Stability Studies • Method Development • Research Formulations Testing capacity:
• Visual Examination • Loss on drying • Deter...
-
Product Extractables & Leachables
There are many contaminants that could be released inside a drug during the production process or by contact with the packaging material. Neotron Pharma will be able to support you from the study of Extractables, to the toxicological evaluation up to the control of the Leachables. What distinguishes us is ...
-
Product Extractable and Leachable Studies
Alcami’s expertise includes the assessment of a broad variety of drug products, routes of administration, and manufacturing system components. A proper assessment includes studies to not only understand which compounds have the ability to leach into the drug product, but also studies to identify which comp...
-
Product Analytical Chemistry Services
Delivering a product from discovery to the market is a long and often difficult process filled with a great number of scientific, safety and regulatory challenges. From early discovery to final product release, SGS offers a broad portfolio of high quality, analytical chemistry services using advanced metho...
-
Product Extractables & Leachables
Extractables & leachables studies enable drug sponsors to quantify and identify the risks of potentially toxic leachable impurities migrating into a drug solution from container closure systems, processing equipment or packaging. Regulatory bodies such as the US Food and Drug Administration (FDA) and T...
-
Product Package and Container Testing
SGS realizes that the package or container that carries your product is vital to its safety and efficacy: a weak container, or one that allows contamination of your product, could put the health of your customers and your reputation at risk. SGS package and container testing services offer a ran...
-
Product Impurities and contaminants analysis
Impurities and contaminants analysis in biotechnology products from SGS – a range of services providing impurities and contaminants characterization in biotechnology derived products and microbial contamination control.To help you meet ICH Q6B guidelines, you need accurate impurities and contaminants analy...
-
Product Biopharmaceutical Development Support Services
Fundamental to your biopharmaceutical chemistry, manufacturing and controls (CMC) development, you will need detailed analytical characterisation data to satisfy regulatory requirements. Our experts strategically deploy orthogonal protein analysis approaches that address key molecular and biological charac...
-
Product Pharmaceutical Product Testing
Element’s pharmaceutical laboratories provide specialist pharmaceutical testing services, including chemical and physical characterization, formulation development, microbial, stability and elemental impurity testing on a wide range of products, from raw materials to finished products.
-
Product Extractables & Leachables Testing
Element's E&L teams use industry-leading expertise, in-depth knowledge of materials and regulatory requirements to help customers assess the potential for leachables, develop strategies to evaluate risk and provide a solid risk-based set of supporting data.
-
Product Extractables and Leachables studies
Extractables/Leachables (E&L) profiles have as a primary purpose identifying any potential chemicals derived from the chemical interactions of a drug with the packaging components ,or the interaction within the pharmaceutical drug formulation.
In addition, E&L is a FDA, ICH, and USP studies...
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
Recommended Products And News
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
















.png)



.png)







.png)

.png)



.jpg)

































-comp270462.png)