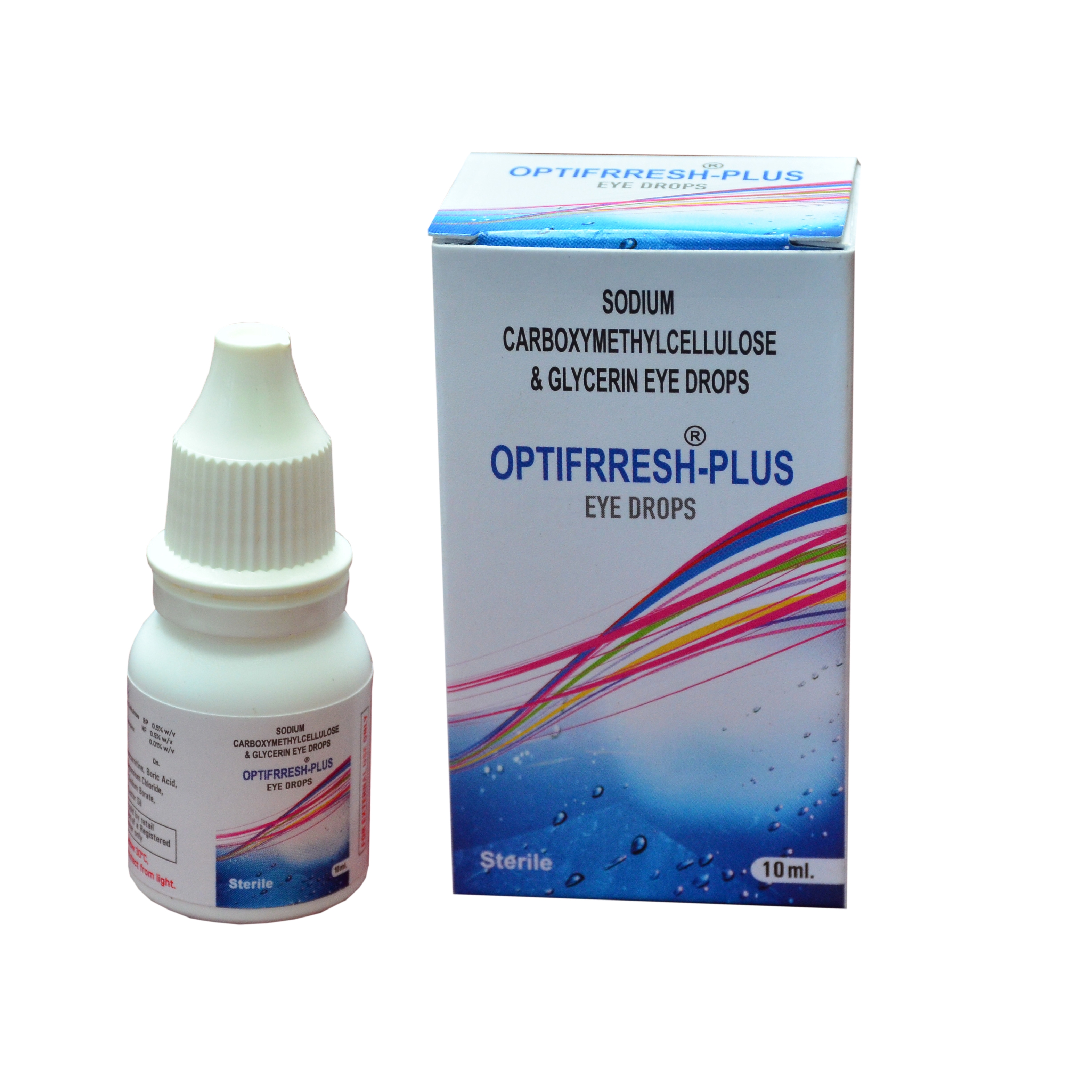
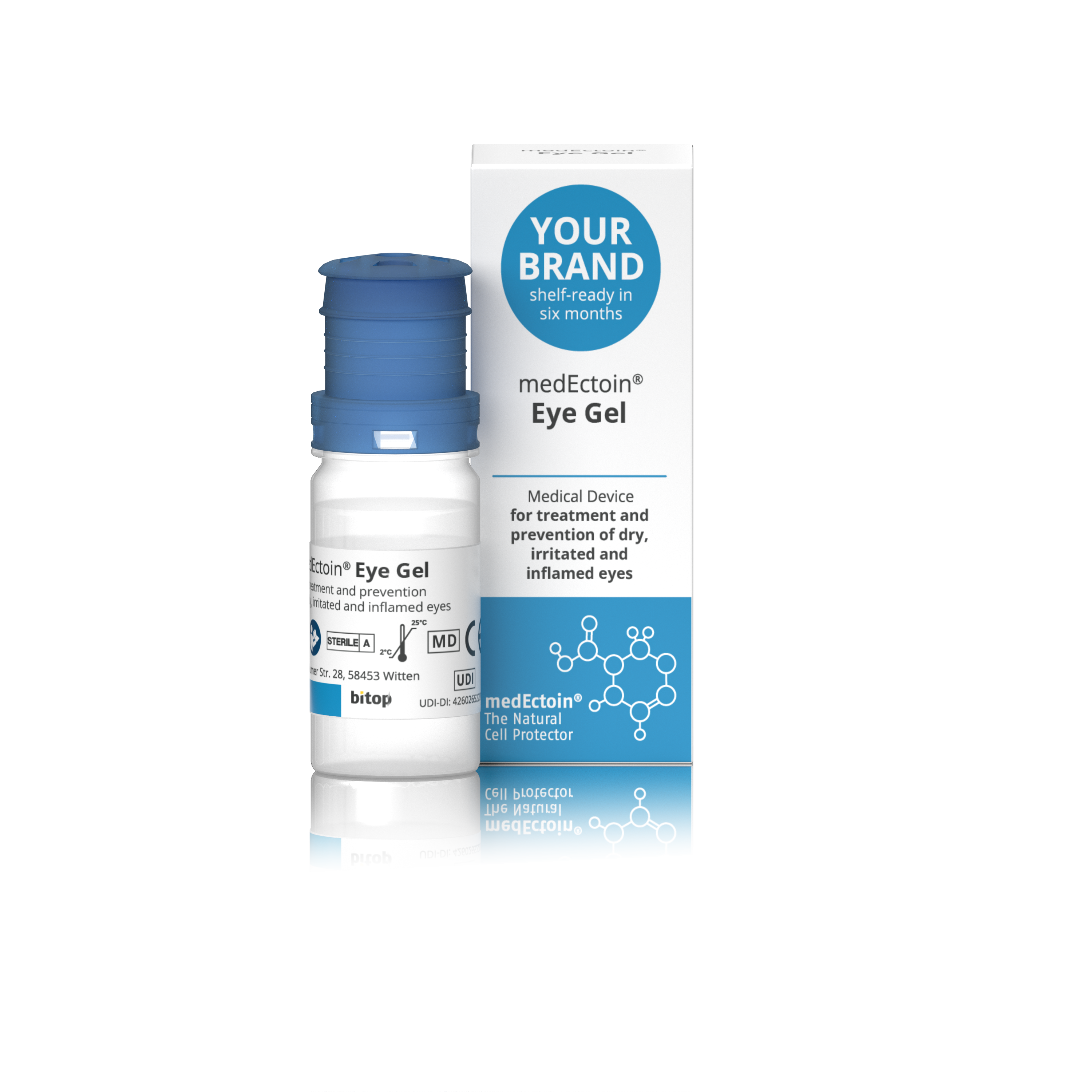
Carmellose Sodium Lubricant Eye Drops CE approved medical device OTC
Product Description
Indiana Ophthalmics

-
IN
-
2018On CPHI since
-
4Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Indiana Ophthalmics

-
IN
-
2018On CPHI since
-
4Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Indiana Ophthalmics (2)
-
Product Carbomer Eye Gel & Hypromellose Night Time and Day Time Eye Gel and Ointment
We have wide range of day time eye gel and night time Lubricant eye gel. These are OTC products with CE approval under medical device for European market.
Carbomer Eye Gel Hypromellose Eye Gel Dexpanthenol Eye Gel Sodium Chloride Eye Ointment
-
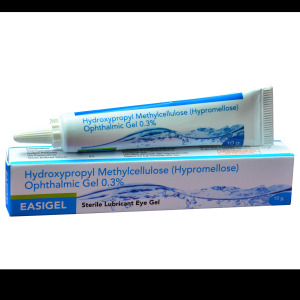
Product Hylofresh Lubricant eye drops CE Medical Device OTC
Sodium Hyaluronate Lubricant Eye Drops
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



























-comp248126.jpg)









































![Toluene sulfonyl methyl cyanide (TosMIC) [36635-61-7]](https://www.cphi-online.com/46/product/129/72/17/inventys-CPHI_Booth.No_Product-36635-61-7(2024Aug22-v0-CT).png)
