Capsugel® Enprotect® capsule
.jpg)
Product Description
Lonza

-
FR
-
2015On CPHI since
-
5000+Employees
Company types
Categories
Specifications
Lonza

-
FR
-
2015On CPHI since
-
5000+Employees
Company types
More Products from Lonza (17)
-
Product PCcaps® capsule
PCcaps® capsules are very small gelatin capsules that are ideal for oral delivery of the neat active in pre-clinical animal studies. They have been designed for performing pre-clinical trials such as pharmacokinetic, pharmacodynamic and animal safety studies. With PCcaps® capsules, there is no need to use ... -
Product Plantcaps® capsule
Plantcaps® capsules, a vegetarian capsule made from pullulan – naturally fermented from tapioca – for a more extensive and distinctive appeal to the booming and discerning healthy lifestyle market worldwide.
Plantcaps® capsules are the premium capsules designed for the needs of the growing and very di... -
Product Vcaps® Capsule
Vcaps® capsules are the first generation vegetarian capsules, meeting the needs of a wide variety of health-conscious consumers. With both KO and OU Kosher and Halal certification, and certified by the Vegetarian and Vegan Action Society, they are an appealing way to address the preferences of an important... -
Product Vcaps® Plus HPMC capsule
An immediate-release HPMC capsule, without gelling agents. Vcaps® Plus capsules offer excellent dissolution profile with pH-independent performance, standard or low-moisture content, great machinability and visual quality. Also available in TiO2 free and with food colorants. -
Product Zephyr(TM) DPI Delivery Solution
Custom inner surface properties, customized moisture levels and more stringent microbial limits all designed to optimize inhalant end-product performance. Available in gelatin, gelatin-PEG, Capsugel® Vcaps® and Capsugel® Vcaps® Plus. -
Product DBcaps® capsule
DBcaps® capsules were developed with a tamper-evident design to specifically address the clinical trial challenges of testing without bias. The wider diameter of opaque DBcaps® allows containment and blinding of large-diameter or uniquely-shaped tablets or other comparator products... -
Product Colorista® capsule
An optimized tool for formulation development enabling compatibility studies before deciding on the final color for the commercial drug products. Available in gelatin and HPMC. -
Product Press-Fit® Enrobing Technology
Press-Fit® Gelcaps utilize a Capsugel® technology that is difficult to mimic and enables brands to create a new product look without having to reformulate their product. Flexible gelcaps are stretched around a caplet of specified shape and dimension using a pat... -
Product Coni-Snap® Sprinkle capsule
Designed to be easily opened by patients and caregivers, these capsules are ideal for pediatric and geriatric populations. Available in gelatin and HPMC. -
Product Coni-Snap® Sigma Series Hard Gelatin capsule
Hard capsules that meet the tightest requirements for visual and print quality though the combination of process control and on-line inspection technology. -
Product Coni-Snap® Hard Gelatin capsule
The reliable and outstanding performance of Coni-Snap® hard gelatin capsules has made them a product of choice for biopharmaceutical companies since their unique locking closure was introduced. Designed and engineered to perform, they are a reliable and widely used capsule. -
Product UC-II® undenatured type II collagen ingredient
The global population continues to age, however mobility is no longer just a concern that affects seniors; consumers across the generations are now looking for efficacious products to promote aging well and support peak physical perfomance. Healthy joints play a pivotal role in protecting mobility as w...
Lonza resources (20)
-
News On track at CPHI Barcelona - The Track Sponsor interview: Lonza
In our packed out content sessions at CPHI Barcelona this year we focus on some of the hottest topics coming up in the pharma industry, with each track sponsored by a leading expert in the field. -
Brochure Capsugel®️ Zephyr™️ Dry-Powder Inhalation Capsule Portfolio
Dry-powder inhalation (DPI) technology offers a favorable drug development opportunity for respiratory or systemic drug delivery. Delivering a uniform dose in a portable, easy-to-use system, capsule-based DPI devices are a simple and cost-effective way to deliver inhalable medication. Capsugel® Zephyr™ is Lonza’s customizable dry-powder inhalation capsule portfolio that is optimized to provide superior performance and compatibility between the capsule/device and capsule/formulation. -
News The Top 5 Industry Content Reads on CPHI Online
If you’re looking for news, product information and market trends from leading pharma companies, the CPHI-Online.com Company Showcases are a great resource for buyers who want to stay up to date, browse product portfolios and find the right partner.The Company Showcase profiles offer a library of free to access, downloadable content, including videos and webinars, reports, whitepapers and product brochures, from the Pharma suppliers and service providers you’d look to meet at our events.
-
Brochure Launch with Lonza™️ innovation services
A full suite of concept-to-market services to turn ideas into breakthrough products. -
Brochure Capsugel®️ titanium dioxide (TiO2) free white gelatin capsules
Discover the possibilities of TiO2-free white gelatin capsules without sacrificing quality and functionality. -
Brochure Capsugel®️ Pharmaceutical capsule portfolio
The Capsugel®️ capsule portfolio provides a wide variety of high-quality products and technologies to help you create a customized solution to bring new innovations to market. -
Video Improve Drug Delivery with Capsule Formulations
In this webinar, formulation scientists and pharmaceutical development specialists will learn how Lonza’s Capsule Applications Services lab can address drug solubility limitations, develop innovative capsules to tailor drug delivery, and optimize encapsulation processes.
Click here to register
-
Video Making a Splash in the US Market: How PBPK Modeling is Playing a Central Role in Risk Reduction
Rapid and efficient development of drug candidates is increasingly important for pharmaceutical companies with accelerated timelines and funding constraints. However, many early drug candidates have poor oral absorption properties making it challenging to achieve target pharmacokinetic (PK) profiles. Without upfront knowledge of absorption risks and mitigation strategies, poor absorption can significantly impact preclinical and clinical study timelines and costs. Physiologically-based pharmacokinetic (PBPK) modeling software, such as the GastroPlus® platform from Simulations Plus, simulates dynamic physiological factors impacting oral performance. When coupled with in vitro measurements, PBPK modeling is effective in early development for 1) identifying absorption risks, 2) assessing the potential for solubility enhancing formulations such as salts, cocrystals, or amorphous solid dispersions to mitigate these risks, and 3) designing and optimizing preclinical and clinical studies with respect to dose, prandial state, or gastric pH modification to maximize the likelihood of achieving desired PK profiles. In this presentation, we will demonstrate how PBPK models combined with Lonza’s custom and off-the shelf in vitro tools and solubility enhancement expertise can be used to identify and mitigate absorption risks in early drug development, reducing the need for drug product reformulation or repeated preclinical or clinical studies. Key Learning Objectives: Learn how PBPK modeling can identify potential oral absorption risks and mitigation strategies (e.g. bioavailability enhancement) for early drug candidates. Learn how PBPK modeling coupled with in vitro testing can guide early selection of drug form and formulation to achieve clinical study goals. Gain insights into how key drug and formulation factors including solubility, permeability, and dissolution rate can impact absorption risks such as poor oral bioavailability, food-drug interactions, and pH-dependent DDI effects. -
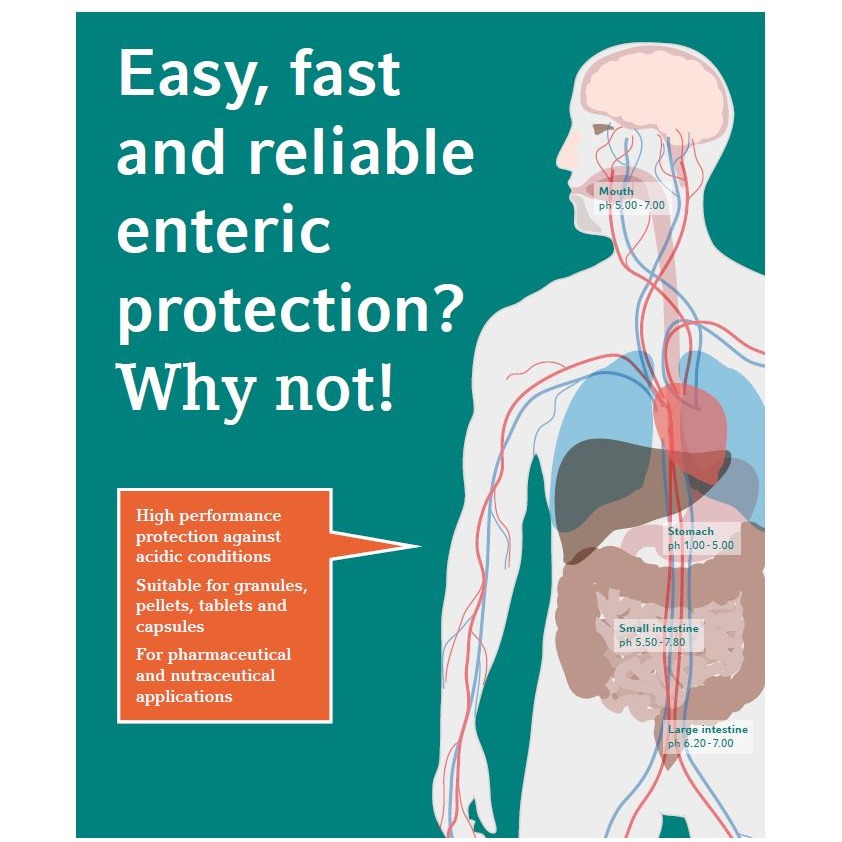
Video The Benefits of the Next Generation Enteric Capsugel® Capsule for your Future Product Development
The next generation enteric capsule enables pharmaceutical companies to develop a targeted delivery solution while simplifying their manufacturing processes.
The solution offers: An acid-protective dosage form with enteric drug release through a patient centric delivery solution; A simple encapsulation process that allows for fast and stable preparation.
This solution is supported by scientific studies, both in-vitro and in-vivo, showing that enteric protection and targeted delivery to the distal intestine are achieved in comparison to marketed references.
Backed up also by performance studies, this capsule showcases excellent machineability and mechanical properties, in line with standard immediate release HPMC capsules. It is available commercially in large quantities.
Join Dr. Vincent Jannin to learn more on the newly launched enteric solution. -
Video Introduction of Psychedelic Therapies into Mental Health - Current Status and Future Perspective
David Erritzøe is an Honorary Clinical Research Fellow at Imperial College London and researches using psychedelics for clinical psychiatric purposes. Due to the history of psychedelics, the subject of David and his team seems a bit controversial. But the research area is blossoming, and the interest in the field is higher than it has been in many years.
In this session, David and Henry Fisher to introduce us to psychedelics and his research on how they might be a key to solving mental illness problems. -
Video Using the Genomic Medicine Toolkit to Develop and Optimize Innovative LNP Formulations
Overview of LNP as an Innovative Approach to Genomic Medicine and Vaccine Development Insights on Core Technologies for Developing RNA-LNP Genomic Medicines and Vaccines: The Genomic Medicine Toolkit Best practices of LNP formulation, optimization and manufacturing Case Study Snapshots: Using the Genomic Medicine Toolkit for the development of Cell Therapies, Gene Therapies, and Vaccine -
Video The Application of EXCiPACT GMP Standard To Pharmaceutical Auxiliary Materials
EXCiPACT is extending its certification scheme to PAMs and is developing a guide for manufacturers and auditors.
As these materials may be in intimate contact with a material which will be administered to patients, they should be manufactured in accordance with GMP principles. Typically, PAMs are removed before use in the manufacture of a drug product or function as processing aids in the manufacture of the excipient.
Although the manufacturing processes used for PAMs may not be traditional chemical manufacturing processes, applying the risk-based approach in EXCiPACT GMP means this standard can be applied to PAMs. The guide will help manufacturers apply EXCiPACT GMP to the preparation and distribution of PAMs and auditors to assess their compliance for certification purposes. -
Video Lessons from the Future: Cannabis and Psychedelic Industries
With North America’s recent embrace of the emerging cannabis and psychedelic industries, there are countless lessons to be learned about how to operate in such an exciting and challenging field. This international and scientifically diverse panel will discuss their cannabis and psychedelic experience in North America to help bolster and harmonize European advancements.
These speakers will share a transatlantic perspective in these emerging medical markets with examples that encompass all aspects of the product development pipeline. Regional specific development challenges, including differences in market preferences, will be highlighted. -
Video Oral Solid Dose Innovation
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. Forefront Key Formulation Variables & Approaches Formulation Development of Complex Oral Dosage Forms Discuss innovations & emergence of advanced technologies (e.g., 3D Printing) Main Driver and Areas of Growth for Oral-Dose Outsourcing Patient-Centric Innovation -
Video The Importance of Colours and Why it Matters in the Pharmaceutical Market
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. The session discusses: Colour in Health products: insights and perspectives The Colour Lab Services and how it can help you define your next coloured product Titanium dioxide discussion and Lonza CHI solutions -
Brochure UC-II®️ Undenatured Type II Collagen
Breakthrough in the competitive joint health & mobility category with UC-II®️ Undenatured Type II Collagen. This joint health ingredient is clinically proven to reduce joint discomfort and increase mobility by helping to repair and rebuild cartilage. -
Brochure The Lonza Engine™ Alliance Program
Our capsule filling equipment partnership program allows customers to match the best equipment for their process with the high-quality and performance of Capsugel®️ capsules. -
Video Value Added Excipients to Unlock the Potential of APIs
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 The humble excipient can play an important role in improving drug formulation, particularly in terms of solubility and stability. This session will explore how to add value to your drug development. Discussion points: The Vital Role Excipients play in producing new drugs and consumer products. An overview of Excipient Quality and Impact on Formulation Excipients Added to Solid Oral Dosage Forms to Improve Drug Solubility and/or Dissolution Latest Developments, demands & trends in the Excipients Market Examples of some Solutions, technologies, and regulatory changes happening on a global scale -
Video Drug Delivery & Development: Overcoming Tomorrow’s Formulation Challenges
This panel aims to provide an overview of the latest approaches to formulation and process challenges for drug development, manufacturing and supply, Highlighting a customized approach to optimize the performance of the end product. Our expert panel members will be sharing their views and experiences on the following key topics: Dry-powder inhalation (DPI) technology for favourable drug development and as a cost-effective and straightforward way to deliver inhalable medication, Drug Delivery Technologies for Women's Health Applications and 3D printing technologies reshaping drug discovery and pharmaceutical development and manufacturing. This presentation was originally aired as part of the CPHI Festival of Pharma. -
Video Pioneering Capsule Innovation: Accelerating Oral and Inhalable Drug Delivery
Explore Lonza new innovation center, dedicated to accelerating capsule development for oral and pulmonary drug delivery. Join us to discover how we foster collaboration with customers and co-innovation in a cutting-edge environment where science meets ingenuity. Learn how our expert team tackles complex formulation challenges, navigates global regulatory requirements, and supports your journey from concept to market. Experience how Lonza can partner with you to redefine the future of pharmaceutical excellence.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










.jpg)












































.png)
-comp248126.jpg)



























.jpg)