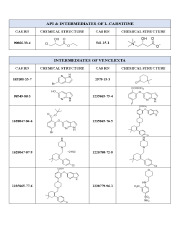
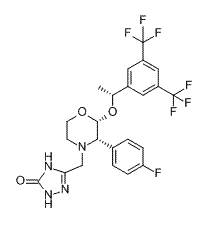
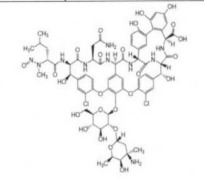
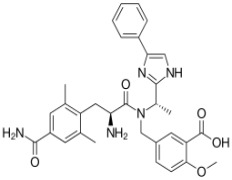
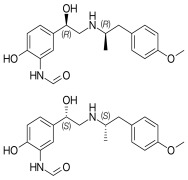
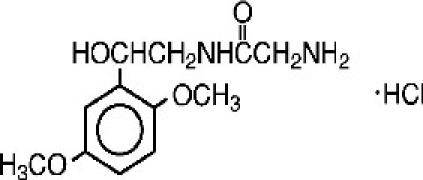
BENDAMUSTINE HCL

Product Description
DCS Pharma USA

-
CH
-
2018On CPHI since
Categories
Specifications
DCS Pharma USA

-
CH
-
2018On CPHI since
More Products from DCS Pharma USA (2)
-
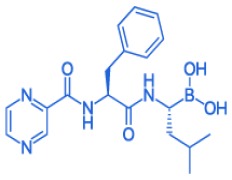
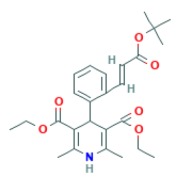
Product BORTEZOMIB
With earlier than expected entry of the generic into the US, the market potential of Bortezomib is expected to increase significantly. Be the first in the market with our technical and regulatory support. REGULATORY STATUS: US-DMF: YES - US-FDA inspection: Q4-2018 - EU-DMF: Submitted Q4-2017 -
Product DAPAGLIFLOZIN
Dapagliflozin inhibits the protein responsible for delivering glucose into the bloodstream. REGULATORY STATUS: US-DMF: YES - US-FDA approval: Expected - EU-DMF: In preparation. www.dcspharma.com/dapagliflozin
DCS Pharma USA resources (1)
-
Brochure PORTFOLIO DCS PHARMA USA
YOUR API EXPERTS: The highest quality, specialized knowledge, and transparency: this is what we bring to active pharmaceutical ingredients.We are your specialists with clearly-defined schedules, extensive high-quality Swiss services, and a global network built up over decades. We focus on a few select therapeutic groups and set the right priorities.Because we’re quality-conscious.
Recommended Products
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






































































%20(1)%20(1)-comp246058.png)