MEGGLE InhaLac 300 Product Information
InhaLac® 300
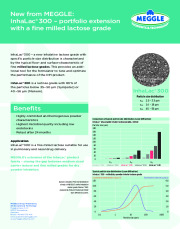
A new inhalative lactose grade with specific particle size distribution is characterized by the typical flow- and surface-characteristic of fine milled lactose grades. This provides an additional tool for the formulator to tune and optimize the performance of the DPI product.
InhaLac® 300 is a lactose grade with 90% of the particles below 35-50 µm (Sympatec) or 40-56 µm (Malvern).
MEGGLE´s extension of the InhaLac® product family - closing the gap between medium sized carrier lactose and fine milled grades for dry powder inhalation.
MEGGLE GmbH & Co. KG

-
DE
-
2015On CPHI since
-
1000 - 4999Employees
Other Content from MEGGLE GmbH & Co. KG (28)
-
Video MEGGLE Spray-dried Lactose
MEGGLE has it.The right spray-dried lactose product for direct compression
Spray-drying (SD) opened the door for lactose in Direct Compression (DC) and had a major impact on tableting technology. After initial quality problems, SD lactose has become a very frequently used, robust excipient grade in pharmaceutical formulation design.
Nowadays, pharma grade lactose is manufactured first, and then converted into SD lactose, avoiding former browning due to the use of a contaminated mother liquor. SD lactose is an excellent example demonstrating the versatility of this material: Besides thermodynamically most stable polymorph α-lactose monohydrate, a precisely defined percentage of amorphous lactose is created in a tailored particle design process using a lactose suspension.
Important: Amount of amorphous lactose
The amount of amorphous lactose can be determined by the lactose dissolved before the atomization step. Generally, SD lactose grades expose between 10 to 15% amorphous lactose, and an aqueous lactose solution contains a majority of ß-anomer, especially at elevated temperatures. This ratio is basically not changed after dispersion, when due to rapid dehydration no formation of a structured crystal lattice is possible. Lactose molecules arrange more the less randomly containing some traces of water. Lack of an ordered structure is seen the reason for its adhesive, more plastically deforming compaction behavior, while sphenoidal, structure-giving, α-lactose monohydrate crystals (also denoted as “tomahawk”-shaped) tend more to consolidate by fragmentation.
Spray-drying: Modulation of porous particles
A combination of these two compaction behaviors favors synergistically tableting and simultaneously loadability of a compact. In a typical SD process a liquid or slurry is rapidly dispersed into a controlled drop size, and its physical shape is preserved by a hot, counter-flowing gas, streaming against the dispersing atomizer. In SD lactose manufacture only aqueous systems are used, drying agent is just hot air. The formation of spherical aggregates of narrow particle size distribution (PSD) impacts powder technological aspects dramatically: Flowability of product is greatly enhanced by spherical shape of agglomerates and smooth surface enabling excellent tablet mass and content uniformity values. Besides that, SD also allows to modulate porosity of particles, which may be applied increasing compaction performance, as well as reducing lubricant sensitivity.
MEGGLE’s spray-dried lactose grades for direct compression are available under the trade names
FlowLac ® 90 and FlowLac® 100 -
Brochure MEGGLE Cellactose Brochure
Cellactose® 80
Lactose and Cellulose are very well established excipients. Both are naturally derived and frequently used as diluent/binder in oral dosage formulations (ODT).
In an effort to obtain synergistic effects, such as improved tablet hardness and adherence capacity, by co-processing both excipients, spray-drying was used in order to bring lactose and cellulose together.
As a result Cellactose® 80, a co-processed excipient, was achieved that provides capability for direct compression, due to given flowability and compactability. Cellactose® 80 is made up of 75 % alpha-Lactose-monohydrate and 25 % powdered cellulose. -
Video MEGGLE Co-processed Excipients
MEGGLE has it. The right co-processed excipients for direct compression
The use of co-processed excipients (CPE) are by far the easiest and fasted way to develop solid oral dosage forms.
The solution: Co-processed excipients
Traditional excipients struggle to cope with current challenges in drug development such as high speed tableting, developing direct compression formulation, low dose related content uniformity issues and insufficient tablet hardness due to high dose loading. Co-processed excipients are in essence the solution to each problem, provided that the right CPE is chosen.
At MEGGLE each CPE was developed having a specific problem in mind, which needs to be solved. Therefore MEGGLE provides a wide range of CPEs. The individual components are of pharmacopoeial quality and are brought together in a sophisticated, controlled manner, resulting in the highest degree of material robustness with regard to their functional related characteristics (FRCs). As quality by design (QbD) gains more and more importance, developers’ need for tightly controlled critical material attributes (CMA) are one of the key benefits of CPEs. The inherent variability of each excipient in a traditional powder blend, which adds up to inherent product variability, can be addressed easily by the use of CPEs, as their manufacturing can be fine-tuned, adding another layer of control, which is impossible to achieve by a simple operation such as blending.
Advantage: Stable ratio of components
Furthermore the ratio of the individual components within the CPE remains stable throughout the entire production process, as these components cannot be separated by physical means. It is important to note that co-processing must not lead to chemical alteration of the individual components.
Important: Full compliance with regularly bodies
Through MEGGLE’s expertise in co-processing, MEGGLE assures full compliance for its CPEs products with regularly bodies around the globe. CPEs are no exception when it comes to its GMP production according to the Joint IPEC-PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients, and USP General Information Chapter 1078. In addition, MEGGLE provides drug master files for all of its CPE, which can be used in conjunction with drug approval by the FDA. It is MEGGLE’s understanding to support its customers from start, providing the first samples, throughout the whole drug development, registration process, and drug product’s life cycle, to finish.
MEGGLE´s co-processed excipients for direct compression are available under the trade names: MicroceLac® 100, Cellactose® 80, StarLac®, RetaLac®, Reta M® and CombiLac® -
Video MEGGLE Agglomerated Lactose - Tablettose
MEGGLE invented in the 70´s the well-known and popular product family Tablettose® – A robust agglomeration technique translates into a robust excipient, which is essential to robust drug manufacturing.
Tablettose® is produced in a continuous manufacturing, a concept which is still quite new for the pharmaceutical industry, but very well accepted and understood by the food industry.
Continuous manufacturing: Reduced production cost:
MEGGLE’s deeply rooted expertise in food production and willingness to conquer the pharmaceutical industry helped a lot to apply the concept of continuous manufacturing to the production of pharmaceutical grade excipients. Not only can MEGGLE take advantage of reduced production cost through continuous manufacturing, which allows for very competitive pricing, a consistent product quality and performance can be achieved through continuous manufacturing.
Agglomerated lactose: Excellent flowability and compactibility
The beauty of agglomerated lactose is its given functionality (excellent flowability and compactibility), while its regulatory status is “save”. Tablettose® complies with all compendial requirements worldwide, which are set for lactose monohydrate. This means, whenever lactose monohydrate is considered as part of a pharmaceutical drug formulation, the drug developer has a highly functional excipient on hand, which’s regulatory status is crystal clear and accepted by the authorities.
Tablettose®: For moisture-sensitive API formulations
Although MEGGLE uses a fair amount of water for lactose’s agglomeration, the final product provides a remarkable low amount of potentially active surface water (less than 0.5 %), which allows for the use of Tablettose® in formulations, which containing moisture-sensitive active pharmaceutical ingredients (API). As lactose monohydrate is non-hygroscopic by any means, stability issues, due to water uptake, should not be of any concern.
The Tablettose® family comprises 3 members to date, each one having its distinct advantages. Tablettose® 80 covers the broadest range of drug formulations and is therefore the first go-to choice when it comes to cost effect and reliable direct compression.
MEGGLE’s agglomerated lactose grades suitable for direct compression are available under the trade names
Tablettose® 70, Tablettose® 80 and Tablettose® 100 -
Video MEGGLE Milled Lactose
MEGGLE has it. The right milled lactose product for wet and dry granulation
In traditional, pharmaceutical dry and wet granulation operation finer particles have proven to be beneficial.
Milled α-lactose monohydrate is available in fine particle size (PS) with narrow particle size distribution (PSD), defined surface properties and density ranges. This allows to modulate in particular a solid formulation’s compactibility, compressibility, porosity, and segregation tendency. Brittle α-lactose monohydrate exhibits good binding properties, and consolidates mainly by fragmentation, but particle flow of milled lactose grades is moderate, depending on size and its corresponding specific surface
Milled lactose grades: A perfect product platform
However, for many important pharmaceutical agglomeration processes (e.g. in economical direct compression) both compactibility and flowability are primordial. Milled lactose grades represent a perfect product platform, for they can be modified greatly, fulfilling the needs of various applications. Impacting particle characteristics by agglomeration has been an early undertaking, where flowability was optimized by a simple and gentle particle size enlargement step, leading into the product family of agglomerated Tablettose®. Dispersion of a milled lactose suspension through a nozzle allows to optimize particle porosity, shape and surface in one step, resulting into the product group of spray-dried lactose (FlowLac® 90/100).
Variety of PSD: Flexibility in tableting
Milled lactose grades also have a long tradition in dry granulation procedures using roller compaction or slugging. These processes consist of a primary agglomeration and a final milling step. In roller compaction due to force feeding reduced powder flow is of minor importance, in some cases low segregation tendency of milled lactose grades may be even seen advantageous for content uniformity reasons. In well-established slugging processes flat compacts are manufactured preferentially avoiding die filling issues. MEGGLE offers a variety of PS/PSD of milled lactose grades offering flexibility in tableting during the second compaction step.
Extrusion/spheronization processes also make use of milled lactose as a filler.
After all, disaccharide lactose may be metabolized by bacteria or fungi and is mainly used in its milled form as bulk substrate/medium for fermentation in bio-industries.
MEGGLE’s milled lactose grades suitable for wet and dry granulation are available under the trade names
GranuLac® 70, GranuLac® 80, GranuLac® 140, GranuLac® 200, GranuLac® 230 and SorboLac®400 -
Video MEGGLE Lactose for Dry Powder Inhalation - InhaLac
MEGGLE has it. The right lactose product for dry powder inhalation
InhaLac® stands for a lactose, which is, in particular, suitable for use in pulmonary and nasal drug delivery.
Inhalation aerosols offer the potential for needle-free systemic delivery of small molecule drugs as well as therapeutic peptides and proteins. An industry standard in dry powder inhalation formulation development, lactose monohydrate is used safely in DPI formulations. Using lactose as an excipient not only improves performance efficiency of the inhaler, but also facilitates powder handling during production. Effective performance of a dry powder inhaler largely depends on the carrier material used for the drug formulation.
Lactose: Carrier of API
Customization has been seen for MEGGLE products, traditionally used for the oral route of administration but is especially important when it comes to the inhalation route of administration, in particular dry powder inhaler (DPI), where lactose plays a predominant role as carrier for the active pharmaceutical ingredient (API) facilitating its delivery to the lower part of lung.
Our Service: Customized lactose for DPI
As DPI development is an interplay between inhalation device, API-properties and lactose carrier, a customized lactose which is tailored in particle size distribution, and surface property, may provide a competitive advantage, when the DPI-drug-development can be achieved in time and on target. Especially increasing the API’s fine particle fraction (FPF) through profound lactose carrier design and subsequently suffering less API loss can be a major improvement and marks successful drug commercialization.
MEGGLE has been active in this field for decades, and therefore has gained a tremendous amount of expertise, which the customer immediately benefits from. It is more than fair to say, that MEGGLE enabled successful products through its customized products for originators and generic companies alike.
Important: Highly controlled production processIn Dry Powder Inhalation (DPI) formulations, the excipient not only acts as a filler but also contributes to the performance features of the DPI. An extensive knowledge of the physico-chemical properties is a prerequisite to guarantee the functionality and safety of the DPI. This includes an established and well-investigated production process. All InhaLac® grades are produced via crystallization and subsequent sieving or milling. The optimized and standardized production process consistently ensures the highest production quality.
With our highly controlled production process different high quality grades for inhalation are obtained, providing an adequate range of particle.
MEGGLE´s lactose grades suitable for DPI are available under the trade names:
InhaLac® 70, InhaLac® 120, InhaLac® 140, InhaLac® 150, InhaLac® 160, InhaLac® 230, InhaLac® 251, InhaLac® 300, InhaLac® 400 and InhaLac® 500 -
Video MEGGLE Sieved Lactose
MEGGLE has it. Lactose for powder preparations.
Although tablets account for the majority of solid oral dosage forms, powder preparations, such as capsules and sachets, present viable options to the developer.
In early stages of formulation development and preparation of clinical trial material, capsules are the ideal choice. When the preservation and long term integrity of the gelatin capsule is needed, lactose is the RIGHT choice! Lactose has limited interaction with gelatin capsules and is the ideal capsule filler for formulators around the world. This clearly has not changed for Hydroxypropylmethylcellulose (HPMC) capsules, which have gained a lot of popularity.
Capsules are of special interest for early stage developments and clinical trials medication, where on a final formulation has not been decided yet.
Trend: Capsule and sachet formulation for children and elderly people.
When developing a powder preparation, processability of the final powder blend, as well as content uniformity and avoiding powder segregation is imperative to a successful development. This is critical for pediatric and geriatric medicines. These patient populations represent a need for enhanced delivery methods that circumvent impaired tablet-swallowing. Capsules and sachets are trending as more popular dosages and offer opportunities for gaining market share.
Key for content uniformity: Suitable particle size distribution as well as bulk density.
MEGGLE has decided to contribute to these needs in a meaningful way by providing a range of lactose grades which differ in particle size distribution as well as bulk density. Both parameters are essential to a successful formulation development. With the right excipient technology choice, content uniformity issues may be a minor obstacle – MEGGLE has it! In order to provide consistent functionality, MEGGLE has implemented a rigorous quality system, which exceeds current Good Manufacturing Practice (GMP).
MEGGLE´s expertise: Off the shelf products or tailor-made sieved lactose grades.
As MEGGLE aims to achieve ultimate customer satisfaction, it has partnered with leading capsule-filling-machine-manufactures in order to provide expertise from both ends; material and processing. By partnering with these companies, MEGGLE is also able to tweak and improve its products, which leads to improved off-the-shelf products but gives the customers also the option to choose a tailor-made grade for even better performance.
MEGGLE´s sieved lactose grades offer great features in high speed capsule filling.
Maximum comfort using MEGGLE lactose in high speed capsule filling is guaranteed. Custom, powder-engineered sieved Lactose grades allow for balancing important powder properties such as density, flow or cohesiveness. In an extensive case study (AAPS 2019) using a MG2 Planeta dosator capsule filling machine, material performance of a formulation comprising low dosage, ultrafine example API Vitamin B2 and coarse, lubricated lactose diluent SacheLac® 80 was investigated. Popular capsule sizes #1, #3, and #4 have been analyzed at 3 discrete filling speeds (15, 38, and 50k capsules/filling unit/h) at different time points according to content and weight uniformity and further performance-related parameters. All results indicated easy capsule filling at high efficiency and quality standards under given equipment design and compendial requirements.
MEGGLE lactose grades suitable for powder preparation e.g. high speed capsule and sachet filling are available under the trade names PrismaLac® 40, CapsuLac® 60, SacheLac® 80 and SpheroLac® 100. -
Brochure MEGGLE Co-processed Excipient StarLac®
StarLac®Lactose and starch are very well established excipients. Both are naturally derived and used frequently in oral dosage formulations (ODT). Lactose is mainly used as diluent. Starch can be used as disintegrant and diluent as well.
In an effort to obtain synergistic effects, such as improved tablet hardness and faster disintegration, by co-processing both excipients, spray-dyring was used in order to bring lactose and starch together. As a result, StarLac®, a co-processed excipient was achieved. It provides capability for direct compression and fast disintegration, that is basically independent from compaction pressure and level of lubricant.
In addition the flowability of StarLac® has been significantly improved over the corresponding physical blend. StarLac® is made up of 85 % alpha-lactose-monohydrate and 15 % white maize starch.
-
Brochure MEGGLE Co-processed Excipient RetaLac®
RetaLac®MEGGLEs co-processed excipient, RetaLac®, appears as a white, or almost white odorless powder, which is freely flowing and partially soluble in cold water. It comprises equal parts of hypromellose (type 2208, a.k.a. “K-type”) with a nominal viscosity of 4000 mPa·s, together with milled ?lpha-lactose monohydrate grade, both of compendial quality. A specialized spray-agglomeration process generates textured, highly structured particles with d50 in the range of many directly-compressible excipients, 100 µm to 200 µm, typically 125 µm.
Lactose identification is performed according to Ph. Eur. lactose monohydrate identification test method C: a red coloration appears. Verification of hypromellose is performed according to the hypromellose monograph, identification test method B, E also Ph. Eur.: a gelation of solution is detectable at >50°C. -
Brochure MEGGLE Anhydrous Lactose DuraLac® H
DuraLac® HDuraLac® H is produced by roller-drying a lactose solution at high temperature to form anhydrous beta-lactose and alpha-lactose crystals at levels approximating 80% and 20%, respectively. During beta-lactose crystallization, no water is incorporated in the crystal lattice resulting in lactose´s anhydrous form.
Subsequent to roller-drying, anhydrous lactose is milled and sieved to the desired particle size distribution, optimizing powder flow and compactability. DuraLac® H deforms by brittle fracture during compaction, it is well-suited for directly compressed and dry granulated formulations (roller compaction, slugging). -
Brochure MEGGLE Tablettose
Tablettose® The product Tablettose® is manufactured by a continous spray agglomeration process, where water is used as the binder and is sprayed onto fluidized fine milled lactose particles, creating liquid bridges to form agglomerated lactose.
Tablettose®, especially designed for Direct-Compression, combines the flowability of coarse lactose crystals and the good compressibility of fine milled lactose.
-
Brochure MEGGLE CombiLac
CombiLac®High-functionality excipient CombiLac® is an integrated, lactosebased, co-processed excipient, specifically designed to ease oral solid dosage form development and manufacture. It comprises 70 % alpha-lactose monohydrate, 20 % microcrystalline cellulose (MCC) and 10 % white, native corn starch, each conforming with Ph.Eur., USP-NF, and JP compendial requirements.
The three individual components are integrated into a monoparticulate structure, which is not separable by physical means. CombiLac® shows improved compaction properties compared to an equivalent admixture of individual ingredients, providing robust tablets with minimal friability.
It assures rapid, hardness-independent tablet disintegration for effective API release, and features powder flow characteristics necessary to enhance dosage form weight uniformity and throughput in DC. -
Brochure MEGGLE InhaLac 140 and InhaLac 150 Product Information
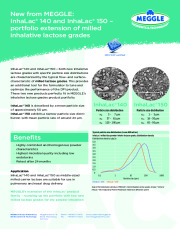
InhaLac® 140 and InhaLac® 150 - both new inhalative lactose grades with specific particle size distributions are characterized by the typical flow- and surface-characteristic of milled lactose grades. This provides an additional tool for the formulator to tune and optimize the performance of the DPI product. These two new products perfectly fit in MEGGLE´s inhalative lactose grades portfolio. InhaLac® 140 is described by a mean particle size of approximately 50 µm.InhaLac® 150 exhibits a narrow particle size distribution with mean particle size of arround 24 µm. MEGGLE´s extension of the InhaLac product family - rounding up the portfolio with two new milled lactose grades for dry powder inhalation.
-
Video MEGGLE The whole world of Lactose
MEGGLE Experts in Excipients
Bavarian-based MEGGLE Wasserburg is one of the world’s foremost experts in lactose and its applications in pharmaceuticals. From its roots as a dairy operation in the late 1880s, MEGGLE has become one of the world’s leading manufacturers of pharmaceutical lactose, supplying the pharma market segment with a broad-based and unique lactose product portfolio.
MEGGLE Excipients & Technology has harnessed outstanding product quality and intelligent innovation to become a global leader in the manufacture of lactose-based excipients, focusing on products for direct tableting and dry powder inhalation with on-going commitment to developing innovative functional products that enable drug development at ease. It supplies products for three main application areas: tableting, powder preparation such as capsules and sachets and dry powder inhalation.
MEGGLE is a pioneer in co-processing technologies that allow simple, robust formulation development and manufacture. Through co-processing, MEGGLE developed highly functional excipients possessing unique qualities for directly compressible immediate and sustained release pharmaceutical solid dosage forms.
A multidisciplinary team of committed and highly qualified people allows MEGGLE clients to benefit from pioneering experience and innovative drive in industrial milk and whey processing. The company constantly strives to develop high-tech, functional products for implementation in customer applications, where they can deliver maximum performance.
Our broad portfolio of products, multiple manufacturing locations, technical centers in major markets, and innovative technologies, make MEGGLE the preferred supplier and valued partner for pharmaceutical product manufacturers.
MEGGLE Excipients & Technologies products:
· Lactose monohydrate
· Anhydrous Lactose
· Co-Processed Excipients
· Lactose for Inhalation
· Lactose for lyophilization and parenteral applications
· Tailor-made lactose products
MEGGLE Excipients & Technologies Services:
· Spray drying
· Co-Processing
· Agglomeration
· Product Customization
MEGGLE Excipient & Technology
www.meggle-pharma.com
service.pharma@meggle.com
-
Brochure MEGGLE CombiLac Brochure
CombiLac®
High-functionality excipient CombiLac® is an integrated, lactosebased, co-processed excipient, specifically designed to ease oral solid dosage form development and manufacture. It comprises 70 % alpha-lactose monohydrate, 20 % microcrystalline cellulose (MCC) and 10 % white, native corn starch, each conforming with Ph.Eur., USP-NF, and JP compendial requirements.
The three individual components are integrated into a monoparticulate structure, which is not separable by physical means. CombiLac® shows improved compaction properties compared to an equivalent admixture of individual ingredients, providing robust tablets with minimal friability.
It assures rapid, hardness-independent tablet disintegration for effective API release, and features powder flow characteristics necessary to enhance dosage form weight uniformity and throughput in DC. -
Brochure MEGGLE DuraLac Brochure
DuraLac® H
DuraLac® H is produced by roller-drying a lactose solution at high temperature to form anhydrous beta-lactose and alpha-lactose crystals at levels approximating 80% and 20%, respectively. During beta-lactose crystallization, no water is incorporated in the crystal lattice resulting in lactose´s anhydrous form.
Subsequent to roller-drying, anhydrous lactose is milled and sieved to the desired particle size distribution, optimizing powder flow and compactability. DuraLac® H deforms by brittle fracture during compaction, it is well-suited for directly compressed and dry granulated formulations (roller compaction, slugging).
-
Brochure MEGGLE Tablettose Brochure
Tablettose®
The product Tablettose® is manufactured by a continous spray agglomeration process, where water is used as the binder and is sprayed onto fluidized fine milled lactose particles, creating liquid bridges to form agglomerated lactose.
Tablettose®, especially designed for Direct-Compression, combines the flowability of coarse lactose crystals and the good compressibility of fine milled lactose.
MEGGLEs Tablettose® 80 was the first available agglomerated alpha-lactose monohydrate of its kind. Its agglomerates possess a size ranging from 0 – 630 µm.
Tablettose® 70 is manufactured using identical starting material as Tablettose® 80; however, the particle size distribution is narrower. The content of fines smaller than 63 µm is reduced significantly and there are no particles larger than 500 µm, making Tablettose® 70 the excipient of choice for narrow particle size distribution and dust free production.
Tablettose® 100 is produced from a smaller starting particle size than the material used for Tablettose® 80 and Tablettose® 70. As a result, Tablettose® 100 has a higher dilution potential compared to Tablettose® 70 and Tablettose® 80 due to increased compactability.
-
Brochure MEGGLE StarLac Brochure
StarLac®
Lactose and starch are very well established excipients. Both are naturally derived and used frequently in oral dosage formulations (ODT). Lactose is mainly used as diluent. Starch can be used as disintegrant and diluent as well.
In an effort to obtain synergistic effects, such as improved tablet hardness and faster disintegration, by co-processing both excipients, spray-dyring was used in order to bring lactose and starch together. As a result, StarLac®, a co-processed excipient was achieved. It provides capability for direct compression and fast disintegration, that is basically independent from compaction pressure and level of lubricant.
In addition the flowability of StarLac® has been significantly improved over the corresponding physical blend. StarLac® is made up of 85 % alpha-lactose-monohydrate and 15 % white maize starch. -
Brochure MEGGLE Reta M Product Information
Reta M®
Reta M® is the first hypromellose/mannitol-based, co-processed excipient specifically designed for DC and dry granulation of modified release formulations. To minimize development time, API dissolution prediction as a function of tablet geometry is possible. This is aided by Reta M®’s dramatic improvement in wettability compared to HPMC alone or in traditional wet granulations and simple admixtures.
MEGGLEs co-processed excipient, Reta M®, appears as a white, or almost white odorless powder, which is freely flowing and partially soluble in cold water. It comprises equal parts of hypromellose (HPMC) with a nominal viscosity of 4000 mPa·s, together with Mannitol. -
Brochure MEGGLE RetaLac Brochure
RetaLac®
MEGGLEs co-processed excipient, RetaLac®, appears as a white, or almost white odorless powder, which is freely flowing and partially soluble in cold water. It comprises equal parts of hypromellose (type 2208, a.k.a. “K-type”) with a nominal viscosity of 4000 mPa·s, together with milled ?lpha-lactose monohydrate grade, both of compendial quality. A specialized spray-agglomeration process generates textured, highly structured particles with d50 in the range of many directly-compressible excipients, 100 µm to 200 µm, typically 125 µm.
Lactose identification is performed according to Ph. Eur. lactose monohydrate identification test method C: a red coloration appears. Verification of hypromellose is performed according to the hypromellose monograph, identification test method B, E also Ph. Eur.: a gelation of solution is detectable at >50°C. -
Brochure MEGGLE MicroceLac Brochure
MicroceLac® 100
Lactose and microcrystalline cellulose are very well established excipients. Both are naturally derived and used frequently as diluents/binder in oral dosage formulations (ODT).
In a effort to obtain synergistic effects, such as improved tablet hardness and adherence capacity, by combining both excipients, spray-dyring was used in order to bring Lactose and Microcrystalline cellulose together. As a result MicroceLac® 100, a co-processed excipient, was achieved, that provides capability for direct compression, due to given flowability and compactability.
MicroceLac® 100 comprises 75 % alpha-lactose monohydrate and 25 % microcrystalline cellulose (MCC), both maintaining their individual chemical identities. -
Brochure MEGGLE InhaLac Brochure
MEGGLE has it.The right lactose product for dry powder inhalation
InhaLac® stands for a lactose, which is, in particular, suitable for use in pulmonary and nasal drug delivery.
Inhalation aerosols offer the potential for needle-free systemic delivery of small molecule drugs as well as therapeutic peptides and proteins. An industry standard in dry powder inhalation formulation development, lactose monohydrate is used safely in DPI formulations. Using lactose as an excipient not only improves performance efficiency of the inhaler, but also facilitates powder handling during production. Effective performance of a dry powder inhaler largely depends on the carrier material used for the drug formulation.
Lactose: Carrier of APICustomization has been seen for MEGGLE products, traditionally used for the oral route of administration but is especially important when it comes to the inhalation route of administration, in particular dry powder inhaler (DPI), where lactose plays a predominant role as carrier for the active pharmaceutical ingredient (API) facilitating its delivery to the lower part of lung.
Our Service: Customized lactose for DPIAs DPI development is an interplay between inhalation device, API-properties and lactose carrier, a customized lactose which is tailored in particle size distribution, and surface property, may provide a competitive advantage, when the DPI-drug-development can be achieved in time and on target. Especially increasing the API’s fine particle fraction (FPF) through profound lactose carrier design and subsequently suffering less API loss can be a major improvement and marks successful drug commercialization.
MEGGLE has been active in this field for decades, and therefore has gained a tremendous amount of expertise, which the customer immediately benefits from. It is more than fair to say, that MEGGLE enabled successful products through its customized products for originators and generic companies alike.
Important: Highly controlled production processIn Dry Powder Inhalation (DPI) formulations, the excipient not only acts as a filler but also contributes to the performance features of the DPI. An extensive knowledge of the physico-chemical properties is a prerequisite to guarantee the functionality and safety of the DPI. This includes an established and well-investigated production process. All InhaLac® grades are produced via crystallization and subsequent sieving or milling. The optimized and standardized production process consistently ensures the highest production quality.
With our highly controlled production process different high quality grades for inhalation are obtained, providing an adequate range of particle.
MEGGLE´s lactose grades suitable for DPI are available under the trade names
InhaLac® 70, InhaLac® 120, InhaLac® 140, InhaLac® 150, InhaLac® 160, InhaLac® 230, InhaLac® 251, InhaLac® 300, InhaLac® 400 and InhaLac® 500
-
Brochure MEGGLE General Brochure
MEGGLE Experts in Excipients
Bavarian-based MEGGLE Wasserburg is one of the world’s foremost experts in lactose and its applications in pharmaceuticals. From its roots as a dairy operation in the late 1880s, MEGGLE has become one of the world’s leading manufacturers of pharmaceutical lactose, supplying the pharma market segment with a broad-based and unique lactose product portfolio.
MEGGLE Excipients & Technology has harnessed outstanding product quality and intelligent innovation to become a global leader in the manufacture of lactose-based excipients, focusing on products for direct tableting and dry powder inhalation with on-going commitment to developing innovative functional products that enable drug development at ease. It supplies products for three main application areas: tableting, powder preparation such as capsules and sachets and dry powder inhalation.
MEGGLE is a pioneer in co-processing technologies that allow simple, robust formulation development and manufacture. Through co-processing, MEGGLE developed highly functional excipients possessing unique qualities for directly compressible immediate and sustained release pharmaceutical solid dosage forms.
A multidisciplinary team of committed and highly qualified people allows MEGGLE clients to benefit from pioneering experience and innovative drive in industrial milk and whey processing. The company constantly strives to develop high-tech, functional products for implementation in customer applications, where they can deliver maximum performance.
Our broad portfolio of products, multiple manufacturing locations, technical centers in major markets, and innovative technologies, make MEGGLE the preferred supplier and valued partner for pharmaceutical product manufacturers.
MEGGLE Excipients & Technologies products:
· Lactose monohydrate
· Anhydrous Lactose
· Co-Processed Excipients
· Lactose for Inhalation
· Lactose for lyophilization and parenteral applications
· Tailor-made lactose products
MEGGLE Excipients & Technologies Services:
· Spray drying
· Co-Processing
· Agglomeration
· Product Customization
-
Brochure MEGGLE FlowLac Brochure
FlowLac®
The product FlowLac® is produced by spray-drying a suspension of fine milled alpha-lactose monohydrate crystals in a solution of lactose. When lactose in solution is spray-dried, a rapid removal of water is taking place, whereby amorphous, non-crystalline lactose is formed in addition to crystalline lactose. Based on the amorphous content, kept on a stable level, non-varying better tabletting properties can be reached. Due to the spray-drying process, FlowLac® has a spherical agglomerate shape, consisting of small alpha-lactose monohydrate crystals bound by amorphous lactose.
FlowLac® 90 was developed to provide greater compactability compared to FlowLac®100 by optimizing the amorphous lactose content. In addition, the particle size distribution makes FlowLac® 90 virtually dust-free.
FlowLac® 100 is the standard grade for spray-dried lactose, which provides an excellent flowability and an extraordinary compactability compared to all other lactose grades.
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance