Audit Activity

Product Description
Asociacion Forum Auditorias (AFA)

-
ES
-
2015On CPHI since
Asociacion Forum Auditorias (AFA)

-
ES
-
2015On CPHI since
More Products from Asociacion Forum Auditorias (AFA) (2)
-
Product Annual Audit Plans
Asociacion Forum Auditorias produces a wide range of audit services which includes annual audit plans. General interest audits are sought and therefore, packages that can be subject to subscription are offered. This optimization formula has been very successful and is repeated every year in what is called:... -
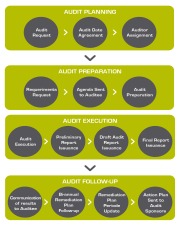
Product Audit lifecycle
Asociacion Forum Auditorias produces a wide range of audit services which includes audit lifecycle. It belongs to audit category. All the steps from audit planning until closing any possible observation detected during the audit execution. The lifecycle can be divided into three big parts with different ac...
Asociacion Forum Auditorias (AFA) resources (1)
Recommended Products
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


































































-comp267516.png)
















-comp323974.png)