- Home
- Mjr Pharmjet GmbH
- Analytical method development
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Analytical method development
.jpg)
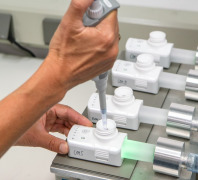
Product Description
MJR PharmJet uses its long gained experience on nano and microparticle formulations to meet your method development needs. We are specialized on the method development of particulate formulations for solid, semi solid and liquid formulations.
Mjr Pharmjet GmbH

-
DE
-
2017On CPHI since
Company types
CMO/CDMO
Contract Service
Contact info
-
-
-
Industriestr. 1b, 66802, Überherrn, Germany
Categories
- Biopharmaceutical Products Analytical Methods (Biopharmaceutical Products)
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Analytical Development
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Development & Validation of Analytical Methods
Specifications
- Supplied fromGermany
Sales Markets
- Middle East Region (e.g. UAE)
- Oceania
- North America (USA, Canada)
- Africa
- Central America (e.g. Mexico)
- East Asia (e.g. China, Japan, Korea)
- Europe - EU countries
- Europe - non EU (e.g. UK, Russia, ex-CIS countries)
- South America (e.g. Brazil, Colombia)
- South Asia (e.g. India, Pakistan, Sri Lanka)
- South East Asia (e.g. Thailand, Philippines, Singapore)
Mjr Pharmjet GmbH

-
DE
-
2017On CPHI since
Company types
CMO/CDMO
Contract Service
Contact info
-
-
-
Industriestr. 1b, 66802, Überherrn, Germany
More Products from Mjr Pharmjet GmbH (2)
-
Product CTM production for nano and microparticle formulations
MJR PharmJet supplies CTM manufacturing services for your nano and microparticle solid and liquid formulations. We evaluate your product and process parameters in detail to ensure the comparability of products before and after CTM production. Furthermore we supply services at every step of technology ... -
Product Extended release microparticle formulations
MJR PharmJet supplies extended release parenteral formulations with biodegradable polymers.
Recommended Products
-
Product PHARMACEUTICAL ANALYSIS DEPARTMENT
Spark-Lab Pharmaceutical Analysis Department performs routine analyses with a special emphasis on determination of excipients, such as:
• Quantitative determination of sorbitol, glycerol, maltitol, mannitol in liquid forms based on our own method; • Quantitative determination of glucos...
-
Product Biotherapeutics
To accelerate the development and marketing of your Biotherapeutics, Quality Assistance offers a complete analytical package to meet the EMA and FDA requirements, all on one site.
Whether it is to extend your analytical capacities or to outsource parts or all of your analytical needs, ...
-
Product Analytical Services
Pharmaffiliates is providing all the Analytical Services (i.e. Method Development, Method Validation & Transfer, Stability Studies, Contract Analysis, etc)
-
Product Analytical Services & EU Batch Certification
Method Development and validation
Stability Studies
European testing & batch certification

-
Product Analytical Services
With a team of experienced scientists and a robust toolbox of analytical techniques and equipment, Experic can support the life cycle of your oral solid dose and/or inhalation pharmaceutical products. Our laboratory staff provides comprehensive analytical solutions to support the formulation, development, ...
-
Product Analytical Development:
•Development and validation of analytical methods to ensure accurate quantification, purity assessment, and impurity profiling.
•Characterization of raw materials, intermediates, and final APIs using techniques such as HPLC, GC, MS, NMR, and XRPD.
•Stability studies and fo...
-
Product Analytical Services
Solvias provides cGMP-compliant contract analytical services to help you ultimately provide safer products to consumers. We provide a comprehensive range of analytical services to the pharmaceutical, biotech, medical devices and cosmetics industry with similar regulatory requirements for raw materia...
-
Product Analytical Development and expertise
Our analytical experienced team support the pharmaceutical development during every phase of the development process. Analytical development transfer and validation, characterization capabilities, stability analysis, ICH Q3D (residuals solvent risk assesment), ICHQ3C (elemental impurities risk assesment...
-
Product Analytical services - OEL: 50 ng/m3
• Control of pharma samples solid state : Identification and quantification of solid state, method development and validation, routine QC. • Analytical techniques available : XRPD, DSC, TGA, DVS, Laser PSD, FTIR, IDR. • All analyses are applicable to toxic samples down to : 50 n...
-
Product Analytical Services
Physico-Chemical testing
- General identification (TLC, HPLC)
- Assay (UV/VIS, HPLC-UV, GC-FID, LC-MS/MS) and dosage uniformity
- Related substances identification and quantification (HPLC-UV, GC-FID, LC-MS/MS)
- Physical determinations (pH, viscosity, density)
- Moisture...
-
Product High-potent Dosage Forms
We have the experience, expertise, and facilities at Sever Pharma Solutions to safely work with your high-potent substances. This includes manufacturing products containing APIs that are ranked in the highest occupational exposure bands (OEB) up to and including OEB6.
Our focus on safety starts ...
-
Product Development
Synerlab provides wide range of services which includes development. Contact us for more information.
-
Product Analytical services
Analytical services
In addition to the basic product quality control, the following connected services are offered, which are required throughout the product development or before the market launch of the respective products:
GMP for excipients, risk assessment...
-
Product CONTRACT MANUFACTURING - SMALL MOLECULES
KD Pharma’s professional expertise, state-of-the-art assets and proven track record in regulatory compliance allow us to become your preferred CDMO solutions partner.
Your Agile Experts
• Responsive to our customers’ needs in a more dynamic customer-centric way • Adaptive to the most diverse re...
-
Product Complex drug formulation
We like to get involved right from the start of a development project and work closely together with our clients and partners on transforming the idea into a product with a solid and commercially viable target product profile (TPP). We set the bar high but always work step by step with a clear focus on...
-
Product ANALYTICAL DEVELOPMENT
TECNALIA, experts in Pharmaceutical Development, Scale-up & Pilot Batches Manufacturing, Clinical Trials and Contract
Manufacturing ANALYTICAL DEVELOPMENT
• Monitoring of the first industrial manufacturing batches.
• Characterization of raw materials according to Pharmacopoeia. q...
-
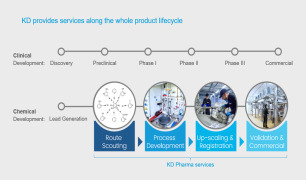
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product CDMO
• Cytotoxic Injection (Liquid , Lyophilized) • ONCOLOGY • CYTOTOXIC API • EU GMP • JGMP (Japan) • KGMP (Korea) • PIC/S
-
Product Bioequivalence tests for topical drugs
We are a GLP lab specialist in vitro permeation testing, including the bioequivalence tests under the EMA draft guideline. We support pharmaceutical companies through bioequivalence studies. Visit our booth and find out more about our R&D dedicated to this topic.
-
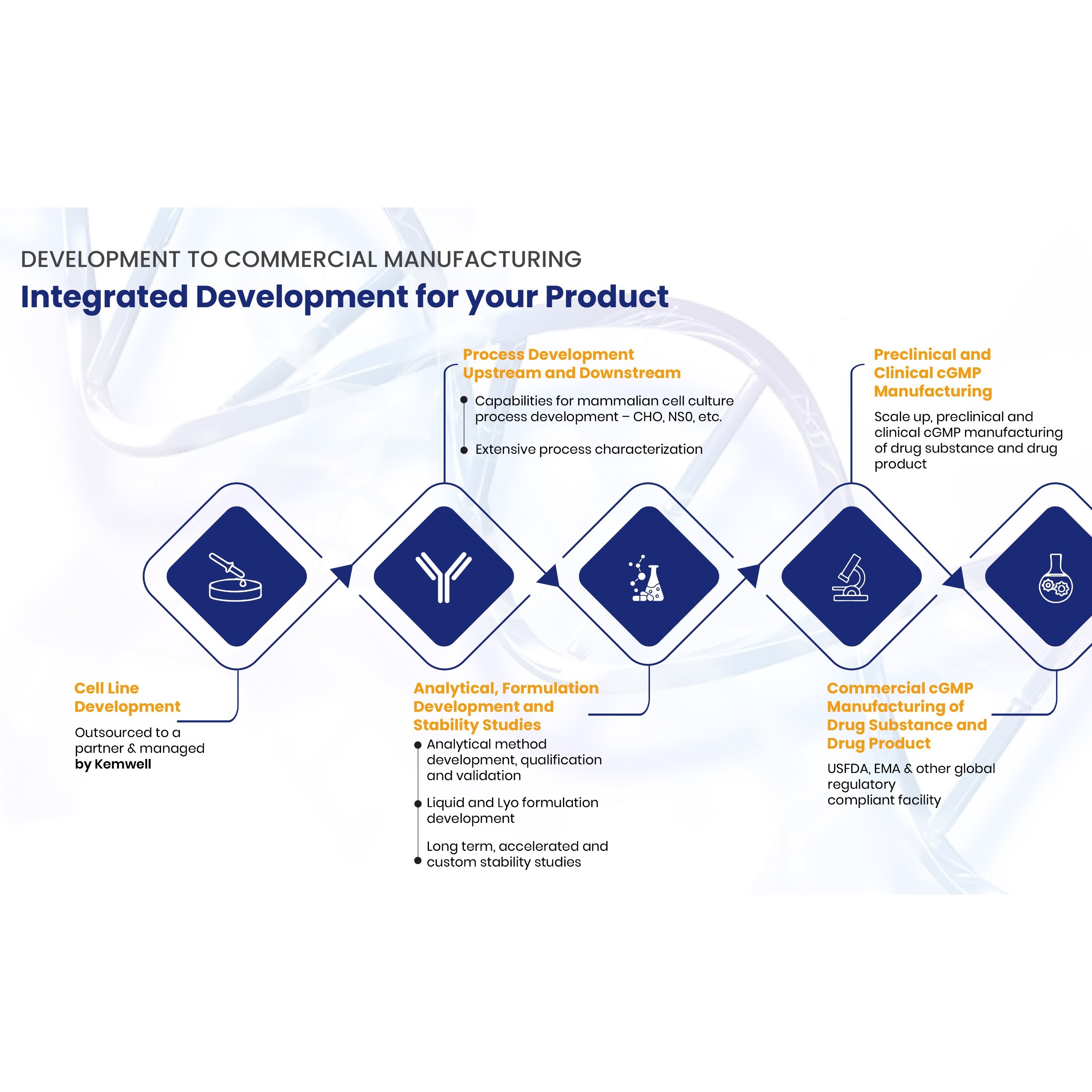
Product Kemwell Biopharma
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa...
-
Product Analytical Development
With top expertise in protein analytics, KBI has successfully completed over 3300 analytical projects for more than 300 customers and more than 130 distinct molecules.
Our experience includes antibodies (IgG1, IgG4, IgM, FAb, ADC, Fc fusion), enzymes, cytokines, growth factors, highly glycosylat...
-
Product Elemental Impurities
Brightlabs has in-house validated methods for the analysis of elemental impurities in accordance to ICH-Q3D. Brightlabs has a unique approach for ICHQ3D, which guarantees quick turnaround times at reduced cost
-
Product GMP Analytical Testing
Singota solutions offers wide range of services which includes GMP analytical testing.
Studies & Methodologies: • Material Identity • In Process • Product Release • Product Specific Assays • Compendial • Microbial (Endotoxin, Bioburden, Sterility) • CoA Generation • ICH Stability (All ICH...
-
Product Pharmaceutical Reference Standards
All Pharmaceutical Reference Standards are supplied with Certified COA along with supporting analytical data like HPLC, Mass, NMR, CMR, UV, IR, TGA, ROI, CHNS.
-
Product Method Validation
Neotron Pharma is able to validate analytical methods developed internally or transmitted by the customer using top of the range equipment such as:
- HPLC-UV / DAD / MSMS
- GC-FID / ECD / MSMS (even in Headspace)
- ICP-MS / AAS / AES / OES
- Post-column derivatization
- Particle si...
-
Product Contract Development Services
Famar R&D is offering a complete set of Contract Development services.
• Formulation and process development for Rx, Gx, OTC, Cosmetics (Solids, Semi-solids /liquids dosage forms, Sterile liquids & Lyophilized powders) and Medical Devices • Analytical methods development and validation&nbs...
-
Product Pulmonary Delivery
Where particle size and performance really matters, Upperton have the expertise.
Drug delivery into the lung is one of the fastest growing, yet technically challenging areas of pharmaceutical development. Producing a successful pulmonary drug product requires expertise in formulation development, part...
-
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.)....
-
Product Analytical Services
ChemCon’s analytics team takes care of your inquiries if you are looking for ICH-compliant quality control, GMP validation, release analysis, impurity determination, reference standards or answers to other analytical queries. Close, outcome-oriented communication is the key to a successful partnership:...
-
Product Research & Development
Avéma offers formulation, process and analytical development support of new or existing products using the most current and effective active ingredients and delivery systems. Our team of scientists bring decades of pre-formulation development expertise to your products, combined with the knowledge of how t...
-
Product Development Service
Lomapharm GmbH provides wide range of services which includes development service. It provides the whole range of control services - from raw material testing to stability testing according to GMP and ICH regulations. One of the main areas of competence and experience is also available for galenic dev...
-
Product Analytical Services - Inhaled Products
With extensive, state-of-the-art analytical testing facilities and equipment, our expert teams are able to develop and validate the methodologies required to characterise inhaled delivery platforms, especially DPI, pMDI and nebulised products.
To ensure seamless support for your development...
-
Product Early Stage Development - Integrated Programs
Accelerating molecules through to proof-of-concept - Simplify early development and accelerate to Proof-of Concept.Proof-of-concept (POC) is a key milestone in the development of a new drug candidate. We understand the transition to POC needs to be fast and cost-effective.With fully integrated capabil...
-
Product A CDMO PARTNER FOR LIFE
FUJIFILM Diosynth Biotechnologies is an industry-leading cGMP Contract Development and Manufacturing Organization (CDMO) supporting the biopharmaceutical industry in the development and production of biologics, vaccines and cell and gene therapies. Our focus is to combine technical leadership in process d...
-
Product Biosafety Testing & Analytical Development Services
Choosing the right partner for analytical and biosafety testing is critical in the race to approval. Our BioReliance® Contract Testing Services offer exceptional, risk-mitigating solutions with technical and regulatory expertise, to help bring life-changing drugs to market.
-
Product Analytical Development
BioDuro-Sundia’s Analytical Testing team offers high quality analytical services including method development and validation, qualification of reference standards, testing and release studies, stability studies, and CMC dossier preparation services. ...
-
Product Development and Validation of Analytical Methods
Analytical methods development and validation: Our method development scientists work with a broad range of products, methods, and analytical technologies (chromatography, mass spectrometry, spectroscopy, biophysical analysis, bioanalytical techniques, etc.) to ensure that the method will meet its int...
-
Product Formulation and Analytical Solutions
GVK BIO offers a range of Formulation R&D solutions that include pre-formulation studies, formulation development, analytical R&D, reformulation and stability studies. We can also support clinical supplies and manufacturing of exhibit batches in collaboration with our partners and offer standalone ...
-
Product Analytical Services (GMP and R&D)
Our analytical portfolio comprises 200+ different techniques – all performed by Coriolis’ experts in-house. Our offering includes:
• Aggregate analytics • Particle characterization • Particle identification • Surfactant characterization • Higher order structure analysis • Funcional assays • Chemi...
-
Product Recipharm Analytical Solutions™
Through Recipharm Analytical Solutions™, we support customers with stand-alone analytical requirements. Our analytical development team has experience from developing hundreds of analytical methods every year, supporting development of formulations ranging from powder in capsules and IV solutions to ER tab...
-
Product CordenPharma Drug Product Support Services
SUPPORT SERVICES FOR OUR CONTRACTED CUSTOMERS • Analytical Development • Validation Support • Regulatory Support • Clinical Supply Services
-
Product GasSpect O2
The Gasporox sensor series GasSpect offers non-destructive Headspace Gas Analysis to be integrated into an inspection machine. The sensors with its highly sensitive laser impresses with performance and precision. The GasSpect series comes in different variations depending on which gas to be inspect...
-
Product Analytical Development & QC
Based on an analytical continuum our testing experts provide solutions all along the value chain from early discovery to clinical trials including method development, validation, quality control, stability studies of the main physicochemical and microbiological testing for innovative and generic products.
-
Product Inhaler Device Testing Services
Through advanced laser imaging techniques, we provide analytical services to support the qualification and validation of spray devices. Testing services on pharmaceutical inhaled drug delivery devices are performed with high-speed imaging to evaluate the dynamic performance of devices by looking at its...
-
Product Reference Standards and NMR Solvents
Clearsynth provides a wide range of high-purity NMR solvents designed to ensure optimal resolution and reliable analysis, supporting both research and quality control applications
Benzene D6 , Chloroform-D , Dimethyl Sulphoxide D6 (DMSO D6) , Methanol D4 , Deuterium Oxide , Tetrahydrofuran...
-
Product Inhalation Test Services
We are the world’s leading provider of orally inhaled and nasal drug product design and development services. We enable the seamless translation of pre-clinical development through to clinical manufacture of OINDPs. We do this through our unique processing technologies and formulation development tools....
-
Product Celanese Development & Feasibility Lab Services
When you work with Celanese you have access to the right mix of services, support, and proven materials to accelerate your drug delivery program through technical feasibility. The Celanese Development & Feasibility Lab offers formulation development, drug release testing, form factor development, mater...
-
Product Extractables and Leachables Testing
Our experts have over 30 years of experience in specialized analytical and toxicology assessment for extractable leachable testing. Intertek offers extractables and leachables testing services through GMP-compliant laboratories located in Whitehouse, NJ (USA) and Basel (Switzerland) wit...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance












.jpg)