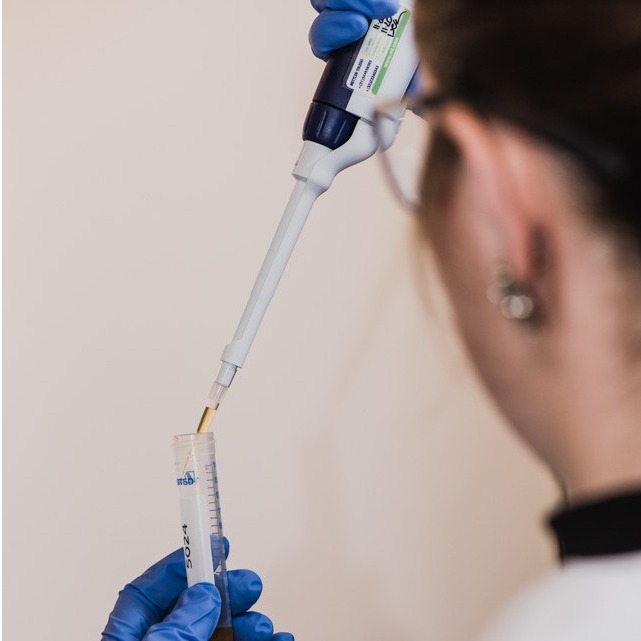
Analytical Development & QC

Product Description
Nuvisan GmbH

-
DE
-
2015On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Categories
Nuvisan GmbH

-
DE
-
2015On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
More Products from Nuvisan GmbH (18)
-
Product Quality Management
Nuvisan GmbH provides wide range of pharmaceutical services which includes quality management. It provides and maintains a comprehensive quality management system to allow each department to manage their project based on processes that have been documented and validated by operations and management. Based ... -
Product Regulatory Affairs
Nuvisan GmbH provides wide range of pharmaceutical services which includes regulatory affairs. Benefits: extensive experience with pharmaceutical start-ups, from contract research organisations to large pharmaceutical companies experienced with a wide range of product types including chemical drug substanc... -
Product Training
Nuvisan GmbH provides wide range of pharmaceutical services which includes training. Pharmaceutical expert with more than 18 years of comprehensive experience (cro and sponsor side) in the field of clinical research (clinical operations, training, coaching, mentoring, quality control, quality assurance) an... -
Product Early Phase Clinical Study Expertise
State-of-the-art phase I/ll unit supporting complex clinical trials • First in Human ( FIH) to Proof of Concept ( PoC) • Clinical pharmacology studies through all phases • In-house safety lab and bioanalytical services • Special patient populations (with a focus on respiratory) • Data m... -
Product Manufacturing, packaging & distribution service
With more than 40 years’ experience as a Clinical Trial Supplies service provider, NUVISAN offers the full range of services in the area of clinical drug supply including: • Regulatory consultancy, CMC support • Non-sterile manufacturing services, including matching placebos • Packaging and lab... -
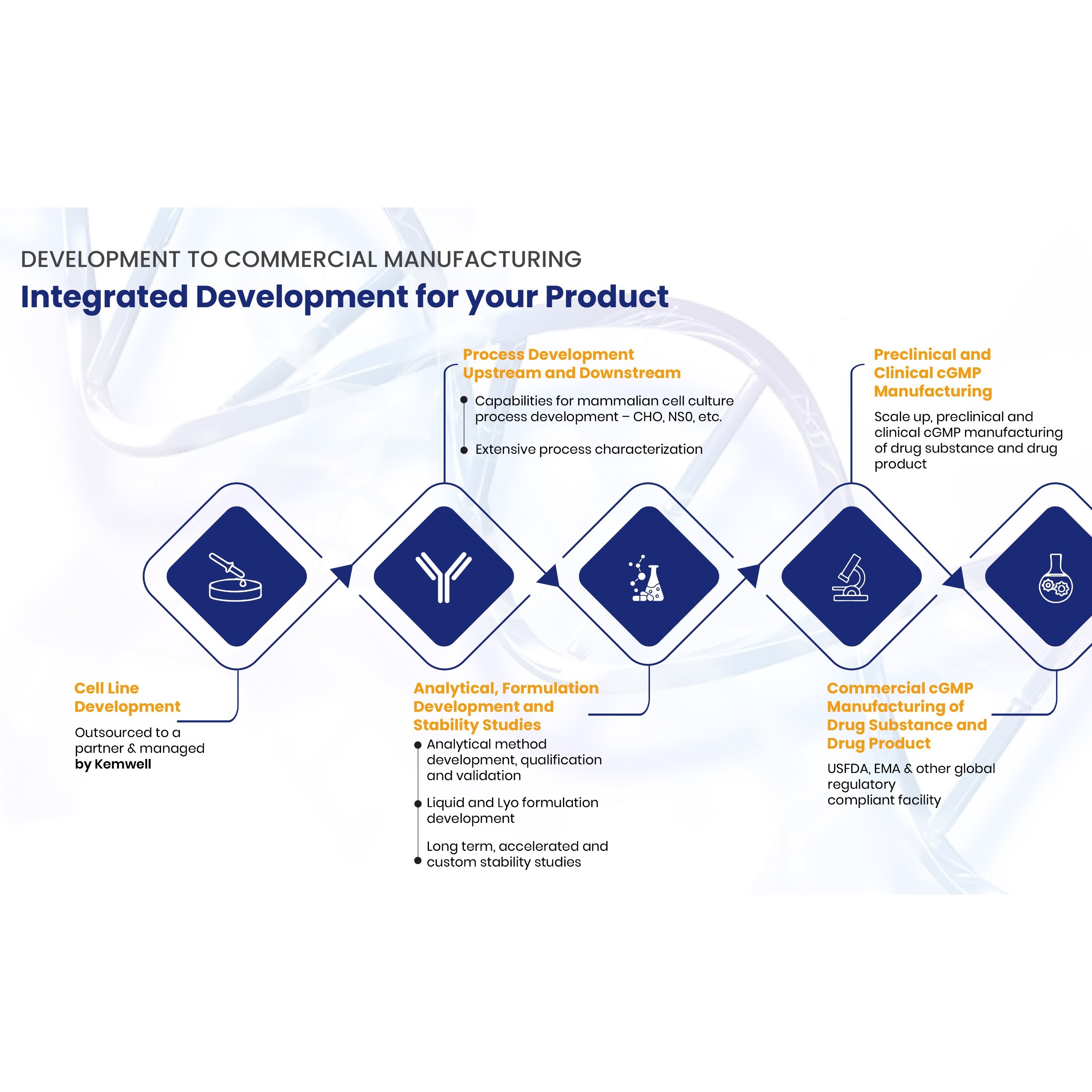
Product Chemical Development
For the pharmaceutical, veterinary or cosmetic industry, our chemists have experience in route scouting and in developing scalable, innovative, sustainable, safe and robust processes up to GMP manufacturing for toxicological and clinical studies. We are also experienced at handling and manufacturing from t... -
Product Clinical Trial Supply
NUVISAN has over 40 years of experience providing clinical trial supplies services, offering a comprehensive range of solutions. These solutions include regulatory consultancy, manufacturing services, packaging and labeling, randomization list creation, analytical testing, just-in-time labeling and distrib... -
Product Formulation Development
The NUVISAN formulation group up has a proven heritage of effective product development with team members being responsible for the creation of topical formulas that supported several commercially successful products. These successes were underpinned by our focus on innovation and patient and health care p... -
Product Bioanalytical Services
To effectively support your drug development project and maximize your R&D productivity, NUVISAN offers a wide array of bioanalytical solutions for small and large molecules. Starting at the discovery, all the way through phase IV, we offer expertise bioassays and high-sensitivity ligand-binding assays... -
Product Formulation Development
Nuvisan GmbH provides wide range of pharmaceutical services which includes formulation development. Service portfolio: formulation development / clinical manufacture; capsules; tablets; granulation / pellets; coating; opcs - powder in the bottle; impd generation: > 55 filings; clinical packaging; phase 1 c... -
Product Immunology
Nuvisan GmbH provides wide range of pharmaceutical services which includes immunology. The testing according to ema guidelines: assay development, validation and glp-compliant performance; screening assays; confirmatory assays assays for neutralizing antibodies; various elisa formats available: bridging, d... -
Product Medical Affairs
Nuvisan GmbH provides wide range of pharmaceutical services which includes medical affairs. Study success is best achieved by a combination of operational excellence, technology and medical oversight. A team of medics, biostatisticians, pharmacovigilance staff and additional experts can cover the full rang...
Nuvisan GmbH resources (6)
-
Brochure Chemical Development Solution
NUVISAN`s scientific team has extensive knowledge in the development of chemical processes up to GMP manufacturing for pre-clinical and clinical studies. We specialize in developing and manufacturing active pharmaceutical ingredients (APIs) and intermediates, offering support from early-stage R&D to commercialization. -
Video Integrated Therapeutic Solutions: Dermatology
At NUVISAN, our passion is creating safe and effective topical products to improve the lives of millions of patients worldwide. Our team of experts is dedicated to drug discovery, DMPK, chemical and formulation development, manufacturing, and clinical development. We create innovative, stable, and elegant topical formulas that have high potential for clinical and market success. With locations in Germany and France, as well as a network of depots worldwide, NUVISAN ensures efficient logistics and support for clinical trial sites globally. -
Brochure ALS‘s Strategic Acquisition Drives Innovation and Science
With ALS's extensive portfolio spanning from drug discovery to post-commercialization, this strategic acquisition offers specific opportunities for our clients -
Brochure Analytical Development and QC Solutions
our testing experts provide solutions all along the value chain from early discovery to post-commercialization. -
Brochure Clinical Trial Supply Solutions
With more than 40 years of experience, NUVISAN provides a comprehensive range of solutions in clinical drug supply including regulatory consultancy, packaging.. -
Brochure Dermatology And Topical Development Solutions
The NUVISAN formulation group has a strong track record of developing effective topical formulas that have supported numerous commercially successful products.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance















































































.jpg)