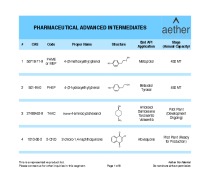
Analytical chemistry&Physico-chemical properties

Product Description
IPO

-
PL
-
2015On CPHI since
-
2Certificates
Company types
Categories
IPO

-
PL
-
2015On CPHI since
-
2Certificates
Company types
More Products from IPO (2)
-
Product Certified toxicological studies
Toxicological tests performed at the Lukasiewicz - IPO:
-allow a toxicological evaluation of chemical substances contained in pharmaceutical products, veterinary medicinal products, plant protection products, food and feed additives, industrial chemical substances, etc.,
-make it possi... -
Product Certified ecotoxicological studies
Ecotoxicological studies conducted by Lukasiewicz - IPO:
-chemical analyses in the range of environmental studies:analysis of concentrations/residues of chemical substances in water, soil, plant and animal material, food, fate and behaviour of chemical substances in the environment
-evaluation ...
IPO resources (1)
-
Brochure Contract research in compliance with Good Laboratory Pracitice
Certified toxicological and ecotoxicological studies
Recommended Products
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













-comp246698.jpg)






































.jpg)













.jpg)