Ampoules Filling
Product Description
Mefar Ilac Sanayii A.S.

-
TR
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Mefar Ilac Sanayii A.S.

-
TR
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Mefar Ilac Sanayii A.S. (6)
-
Product Blow fill seal
Mefar offers wide range of products which includes blow fill seal. We have aseptic filling of 5ml to 50 ml, available in twist off seal or screw cap PE containers.
Mefar also offers strip filling for nebule type of products.
Mefar's all services have EMA certification.
... -
Product Lyophilization
Mefar offers wide range of products which includes lyophilization in vials with its 20 million + capacity with its state of the art freeze dryers.
Mefar's all services have EMA certification.
Contact us for more information. -
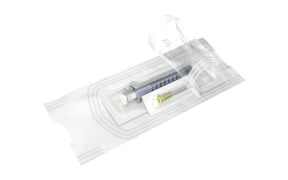
Product Pre-filled syringes
Mefar offers wide range of products which includes pre filled syringes with over 120 million capacity between 0,5 ml to 50 ml.
Mefar's all services have EMA certification.
Contact us for more information. -
Product Vials
Mefar ilac sanayii offers wide range of products including vial filling between 2 ml through 300 ml.
Mefar's all services have EMA certification.
Contact us for more information. -
Product Analytical Services
Mefar offers wide range of analytical services which includes process validation / scale up batches / stability studies / analytical method transfers and more.
Please contact us for more information.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











.jpg)















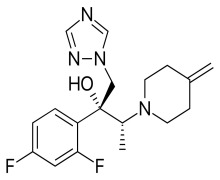








-comp247874.png)



















































.jpg)