Aizon Predict

Product Description
Aizon

-
US
-
2023On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Categories
Aizon

-
US
-
2023On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
More Products from Aizon (2)
-
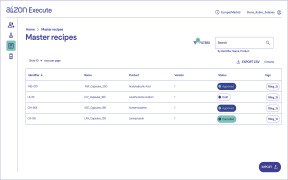
Product Aizon Execute
Intelligent Batch Recording: go fully digital in just 6 weeks
- Automated recipe conversion
- Implementation & validation without the heavy lifting
- Easy workflow management
- Intuitive GMP environment (easy change management)
- GxP by design: we are pharma-only
- Process data unlock... -
Product Aizon Unify
Unify is our Contextualized Intelligent Lakehouse: Streamline pharma manufacturing analytics by integrating your data across your processes, systems and sites and leveraging solutions made for the industry.
Aizon resources (1)
-
News Aizon Launches New AI-powered eBR Light Solution to Enhance Manufacturing Productivity in Collaboration with Euroapi
SAN FRANCISCO- Aizon, an Artificial Intelligence (AI) SaaS provider dedicated to transforming pharmaceutical manufacturing operations, is launching a new product called Aizon Execute, an innovative eBR (Electronic Batch Record) software product, developed in collaboration with Euroapi, a leading global API manufacturer.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance









.jpg)