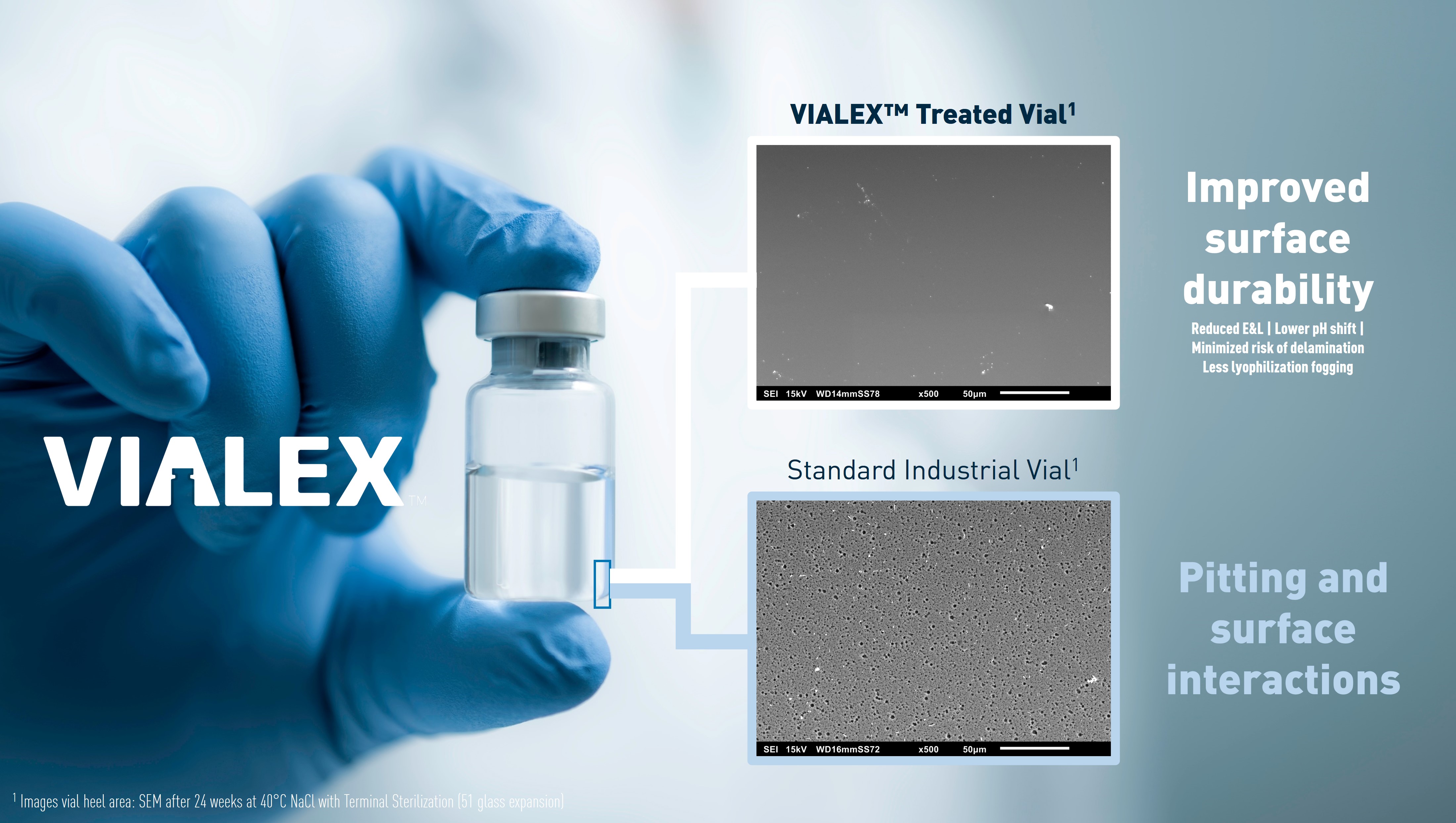
Aenova CDMO Manufacturing Services INtro
Product Description
Aenova

-
DE
-
2015On CPHI since
-
4Certificates
-
1000 - 4999Employees
Company types
Categories
Specifications
Aenova

-
DE
-
2015On CPHI since
-
4Certificates
-
1000 - 4999Employees
Company types
More Products from Aenova (12)
-
Product ACETYLCYSTEINE 600 mg effervescent tablets (aluminium-tubes)
Effervescent tablets to liquefy mucus and to facilitate expectorationin bronchitis induced by a cold. White, fl at, round tablet, breaking notch on one side, characteristic odour ofacetylcysteine and lemon aroma, diameter 18 mm. -
Product Aenova Softgel Manufacturing Services
Aenova has more than 35 years of experience in the formulation and analytical development and production of soft gelatin capsules.
Our production facilities are equipped with state-of-the-art equipment to process any formulation and any capsule design, color and size.
With development... -
Product Aenova Sterile Fill Finish & PFS manufacturing
The Aenova Group is a premier solution partner for sterile dosage forms with 3 FDA approved facilities offering high quality injectables for human health and animal health products.
Our Sterile services include specialized capabilities for:
Beta-lactams (penicillins, cephalosporins)In... -
Product ARTICHOKE (CYNARA SCOLYMUS) sugar-coated tablets
Artichoke 300 mg for the symptomatic relief of digestive disorders, such as dyspepsia with a sensation offullness, bloating and fl atulence.
Dossier in EU-CTD available. Product registered in Germany (Galle-Dragee with Artichocke) and UK (DigestAid coatedtablets), complies with HMPC-Monogr... -
Product BEARBERRY LEAF (ARCTOSTAPHYLOS UVA-URSI) film-coated tablets
Bearberry leaves film-coated tablets are used for inflammatory diseases of the urinary tract.
The Dossier in EU-CTD format available -
Product ECHINACEA (ECHINACEA PURPUREA) soft gelatin capsules
ECHINACEA 176 mg is used for the short-term prevention and treatment of common cold. Dossier in EU-CTD availableProduct registered in Germany and UK. -
Product GINKGO (GINKGO BILOBA) film-coated tablets
Improvement of brain defi ciency.
Dossier in EU-CTD available -
Product HAWTHORN (CRATAEGUS SPP.) sugar-coated tablets
Support the heart and cardiovascular function.
Dossier in EU-CTD available. Product registered in Germany (Weissdorn-Dragees). -
Product MILK THISTLE (SILYBUM MARIANUM L.) soft gelatin capsules
Relief of digestive disorders, sensation of fullness and indigestion and to support theliver function.
Dossier in EU-CTD available. Product registered in Germany (Mariendistel Kapsel); complies with HMPC-Monographfor Silybum marianum, fructus -
Product OPIPRAMOL DIHYDROCHLORIDE film-coated tablets
Generalized anxiety, somatoform disturbances
Modules 2-5 in EU-CTD availableBE study of Opipramol 50 mgBio-waiver for Opipramol 100 mg -
Product VALERIAN (VALERIAN OFFICINALIS) / HOP (HUMULUS LUPULUS) sugar-coated tablets
Relief of mild symptoms of mental stress and aid to sleep.
Dossier in EU-CTD available. Product registered in Germany (Beruhigungs Dragee mit Baldrian Hopfen). Complies with HMPC-Monograph for Valeriana officinalis L., radix and Humulus lupulus L., flos -
Product VALERIAN (VALERIAN OFFICINALIS) sugar-coated tablets
Relief of mild symptoms of mental stress and to aid sleep.
Dossier in EU-CTD available. Product registered in Germany (Nachtruhe Baldrian Schlaf-Dragees N); complies with HMPC monograph for Valerian officinalis L., radix (Valerian root)
Aenova resources (18)
-
News Aenova scales up high-potent medicines´ development and manufacturing capabilities
Aenova, one of the world's leading Contract Development and Manufacturing Organizations (CDMO) expands its offering for the development and manufacturing of high-potent active pharmaceutical ingredients at its Regensburg site. Aenova thus provides its customers with doubled capacities for laboratory and manufacturing and strengthened its development services for innovative APIs including pre-formulation services. -
Brochure Aenova Corporate Brochure
We go beyond manufacturing with a commitment to quality and reliability that is anchored in everything we do. Scientific expertise, innovative technology platforms and a strong customer focus make us the one-stop-shop of choice. -
News Aenova expands with new gummy line for pharma and food grade
Aenova, one of the world's leading CDMOs (Contract Development and Manufacturing Organizations) for the pharmaceutical and healthcare industries, is implementing a new gummy line at its site in Cornu, Romania. Gummies are quickly emerging as the dosage form of choice for many food supplements and even pharmaceutical applications. The new gummy line will be launched by the end of 2024 with a capacity of approximately 1 billion gummies per year. -
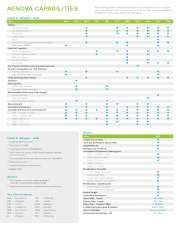
Brochure Aenova Capabilities
Aa a leading global contract manufacturer and development service provider with 14 production sites in Europe and the USA, we offer extensive capabilities for Steriles, Semi-Solids, Solids and Packaging. -
News Aenova white paper: Cannabinoids: Therapeutic effects and the advantages of soft capsules as appropriate dosage form
Cannabinoids can provide great benefits for the treatment of cancer and central nervous system diseases. Softgel capsules are the preferred delivery form for cannabinoid products for many reasons. Aenova presents this in the new white paper "Cannabinoids: Therapeutic effects and the advantages of soft capsules as appropriate dosage form".
-
Brochure Aenova HPAPI Brochure
There is nothing as important as experience when it comes to handling highly potent active pharmaceutical ingredients (HPAPI) – from product development to commercial supply. At Aenova we offer specialized, modern infrastructure and highly qualified experts for successful development and production of highest quality HPAPI products. -
News Aenova white paper: Faster time to market for pharmaceuticals using capsule low-dosing technology
One of the biggest challenges in pharmaceutical research and development is to complete development projects as quickly as possible. More and more drugs fall into the category of 'unmet medical need' and therefore qualify for accelerated approval. But developers need time to optimise the formulation of the sensitive or highly potent compounds that dominate the current pipeline of drug candidates.Finding the right balance between speed and thoroughness is a challenge. But it can be solved by choosing microdosing technology in capsules. Manufacturers can dose small amounts of pure active pharmaceutical ingredients (APIs) or mixtures directly into hard capsules. Microdosing of less than 5 mg or low doses of 5 mg to 100 mg in hard capsules is a good option that allows manufacturers to get new drugs into research and development or onto the market faster.The direct, precise and reliable filling of low-dose hard capsules can be scaled up in an industrial process. -
Brochure Aenova Animal Health Broschure
Aenova offers over 20 years of experience and the full services of 7 approvedmanufacturing sites. This enables us to provide excellent end-to-end services fromdevelopment to packaging for a wide range of medicines for companion animals and livestock. -
News New Aenova White Paper: Softgel Capsules. A solution for poorly soluble and low bioavailability APIs
Low aqueous drug solubility in pharmaceutical development is a recurring issue. The softgel capsule offers many possibilities in the development of new drugs, especially when it comes to solubility and bioavailability enhancement. In the current white paper, experts from Aenova explain the challenges of softgel capsules in development and commercial production, particularly considering the complexity of softgel production technology, as well as the suitability of the softgel capsule as an appropriate dosage form for certain areas of application.
Explore the transformative potential of softgel capsule technology with the new Aenova white paper.
This white paper delves into the intricacies of developing and producing softgel capsules, highlighting the advantages they provide in drug formulation, including improved stability, patient-friendly administration, and precise dosing.
-
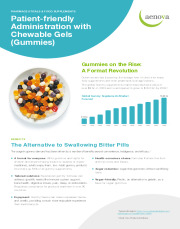
Brochure Aenova Gummies Onepager
Gummies are fast becoming the dosage form of choice for manyfood supplements and even pharmaceutical applications.The global Gummy supplements market has reached a value ofover $9 bn in 2023 and is anticipated to grow to $18.2 bn by 2033. -
News Aenova white paper on "Development of highly potent medicines”
HPAPIs (highly potent active pharmaceutical ingredients), provide precision treatment for cancer, autoimmune disorders, infectious diseases, and other medical conditions. Their safe handling is a challenge for drug manufacturers, who have to meet equipment, environmental and product quality requirements in the production and development of HPAPIs. -
Video Jan Kengelbach, CEO of Aenova, talks about three key elements of how Aenova enables its costumers to grow their business
Jan Kengelbach, CEO of Aenova, discusses how the company’s partnership with Kühne Holding AG, its focus on capacity, innovation, and flexibility, and its strategic growth plans position it as a leading partner in the CDMO sector. He also shares insights on Aenova’s approach to evolving industry trends and attracting top talent. -
News Aenova publishes white paper on the "Safe and efficient handling of high potent drug products"
High-potent drug products for oncology or hormone therapy will play an important role in innovative drug therapy in the future. The development and production of drugs with high-potent active ingredients is highly complex and often must be implemented with acceleration. Handling of high potent solid dosage forms therefore is a key need of current pharmaceutical development and manufacturing. To ensure safe and efficient handling of high potency products, mastering a holistic production approach is essential. This drives a fast time-to-market for your new high potent product.
-
Brochure Aenova CDMO Services
Aenova is a leading contract development and manufacturing organization (CDMO) for the pharmaceutical and healthcare industry worldwide. The range of services covers the entire value chain of development and production of all common dosage forms (solid, semi-solid and liquid, sterile and non-sterile; high potency and low dosage) and product groups in drugs and food supplements for human and animal health. Aenova offers its customers full service, ranging from product development to the purchase of raw materials, production, analytics, market release. Aenova develops, produces and packages at 15 locations in Europe and USA. Around 4,500 employees contribute to the success of Aenova. www.aenova-group.com -
Video CPHI Milan on site interview with Aenova CEO
Jan Kengelbach, CEO of Aenova, talks about three key elements of how Aenova enables its customers to grow their business.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance















































































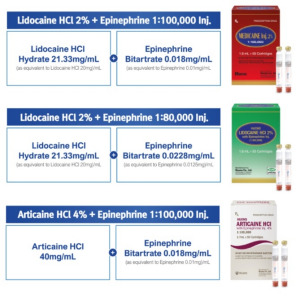






























-comp301214.png)





-comp247874.png)


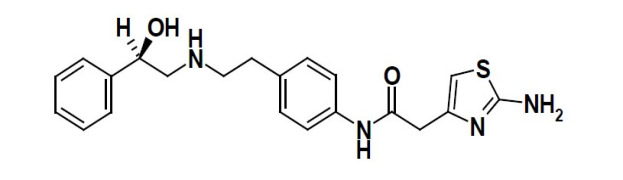






-comp273426.jpg)