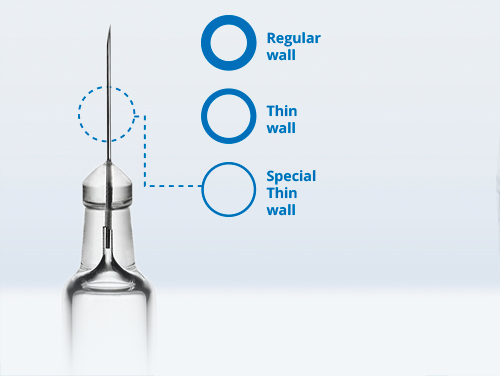
Collaborating to Simplify Device Development and Optimize Glass Solutions for Auto-Injectors

Advances in auto-injector design now provide the option to use both 1ml and 2.25mL syringes plus employ technology to adapt to varying fill volumes and viscosities all whilst maintaining the same device form. This provides a solution for the formulation changes that take place during drug development, clinical trials, and life cycle management. When looking at the performance of any parenteral injection device, the drug containment systems play a crucial role. Auto-injector performance and functionality is strictly linked to key dimensional tolerances of glass syringes. We will illustrate the importance of syringe design to ensure these requirements and how monitoring dimensional parameters at an early stage of the development is key to ensure the intended use and ultimately work to preserve patients’ safety. Benefits of tight collaboration between device designer and manufacturer will also be discussed.
Stevanato Group

-
IT
-
2015On CPHI since
-
5000+Employees
Other Content from Stevanato Group (20)
-
News Pharmapack 2024 - From the Floor
Paris once again welcomes Europe’s leading trade show in pharmaceutical packaging and drug delivery innovation. Join our content team as Pharmapack 2024 opens its doors to leading experts and innovators in pharmaceutical packaging and drug delivery. -
Video Modular and flexible Assembly Equipment
Assembly Equipment combining transport platforms and process modules: an offer of manual, semi-automatic or fully automatic assembly lines customized to your device program. -
News Pharmapack 2024 – Stevanato Group talks sustainable solutions
This January Pharmapack Europe is once again bringing the epicentre of all things pharmaceutical packaging to Paris, France. This year, to enhance our sustainability offering at the event, we are partnering with Stevanato Group. In the following interview Stevanato Group explains why sustainability is so important to them. -
Video Pen Injector Assembly Line
A flexible mid-high speed pen injector line assembling different formats and integrating extensive in-process controls. -
News Exclusive Collaboration Agreement for the Innovative Aidaptus® Auto-Injector
The Aidaptus® disposable auto-injector platform, designed by Owen Mumford, will be jointly offered to global biopharma companies to support their drug development programs with engineering and manufacturing support provided by Stevanato Group. -
Brochure Alina®: User-friendly disposable pen injector platform for variable and multi-dose treatments
Alina® is a variable-dose pen injector developed by Stevanato Group based on Axis-D intellectual property and technology exclusively licensed from pen injector device expert Haselmeier, for the treatment of diabetes, obesity, and other therapeutic areas. -
News Stevanato Group and Gerresheimer AG present EZ-fill Smart, a new and innovative Ready-To-Use Vial Platform
Leveraging the established EZ-fill® market leading technology, process, and product optimisation to improve quality, reduce total cost of ownership, and shorten lead times. -
Whitepaper GMP Annex 1 Implementation
How RTU vials and cartridges can be a suitable, time-efficient and cost-effective answer to meet new regulation requirements. -
News Stevanato Group presents Vertiva™, a versatile on-body delivery system platform for a wide range of injectable therapies and for large delivery volumes (up to 10mL)
The Company offers the platform as a readily customizable pre-filled and pre-loaded on-body solution for drug delivery. Device development is making significant progress, with samples expected to be available in 2023. -
Whitepaper mRNA therapies on the rise
Learn more from this White Paper providing insights on tests and solutions performed on primary packaging to overcome mRNA challenges.
-
News Growth opportunities abound for auto-injectors amid patient-centric healthcare transition, experts tell CPHI webinar
Tremendous growth opportunities exist for auto-injectors as their value is increasingly being recognised across several therapy areas, experts have told a recent CPHI webinar, sponsored by Stevanato Group. -
Webinar CPHI Webinar Series Optimising Pharma Visual Inspection - Unlocking the Potential of AI
Artificial Intelligence (AI) has triggered a fundamental shift within the innovation paradigm for the pharmaceutical industry and has been implemented in almost every aspect of the value chain, from drug discovery and development to manufacturing. One application of AI which offers particular value is the realm of visual inspections, which present a challenging step in the packaging process. This is particularly true for products with complex characteristics where manual inspections can sometimes fall short, leading to errors and inconsistencies. AI learning models can optimise the packaging process by detecting minute errors, resulting in fewer rejects, less intervention and a more efficient process overall. This webinar will tackle some of the key drivers and benefits to implementing AI for pharma visual inspections to enhance performance, improve accuracy, efficiency and overall quality. -
News WEBINAR: Early stage considerations for the manufacture and delivery of vaccines: Watch Now!
Watch this recent webinar exploring the challenges of distributing COVID-19 vaccines to a global population and the role the packaging sector is playing to ensure safe and speedy distribution of shots -
News Stevanato Group and Bexson Biomedical announce collaboration to develop a customized wearable ketamine delivery device
Patients suffering from chronic and acute pain disorders will benefit from a convenient, discreet, and easy-to-use device delivering a novel alternative to opioid-based pain management. -
Brochure Vertiva®, Next-generation On-Body Delivery System
In this article, Stevanato Group, introduces Vertiva®, the company’s next-generation on-body delivery system platform, and discusses how it meets the needs of pharma companies looking to deliver large-volume doses in a patient-centric manner. -
News Stevanato Group to open US Technology Excellence Center in Boston
Due to begin operations in late September, the US TEC will bring value-added laboratory services to the heart of the biotech community. -
Brochure Innovative Needle Geometry Accommodating Viscous Biologics
In this article, Stevanato Group discusses the challenges faced in administering highly viscous biologics to patients via subcutaneous injection and how its special thin-wall needles can tackle them. -
News Stevanato Group Launches Robotic Human-like Inspection Unit with AI-based Machine Learning Capabilities
The Vision Robot Unit will provide biopharma companies with a fully automatized, highly accurate and flexible inspection system equipped with artificial intelligence for a variety of drug products, for increased operational efficiency. -
Brochure A forensic approach for combination devices
Discover how Stevanato Group helps pharma companies identify relevant testing to de-risk the product development lifecycle and quickly uncover the root causes of product failures. -
News Supporting global industrial scale-up at a pandemic speed with Type 1 pharma glass containers and timely solutions
In a compressed timeframe, producing large quantities of pharmaceutical glass containers to deliver the coronavirus vaccine around the world is a critical factor for a successful global response. Type 1 glass is a proven and widely available solution for pharma glass containers that preserves the stability of vaccines. Players in the pharmaceutical supply chain are committed to supporting pharmaceutical companies and governments in the fight against the pandemic.
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance