Acenocoumarol
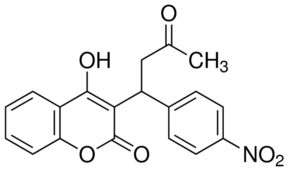
Product Description
Polpharma S.A.

-
PL
-
2015On CPHI since
-
4Certificates
-
5000+Employees
Company types
Specifications
Polpharma S.A.

-
PL
-
2015On CPHI since
-
4Certificates
-
5000+Employees
Company types
More Products from Polpharma S.A. (34)
-
Product Sitagliptin hydrochloride Polymorphic form III
DISCLAIMER
The above product is presented solely for informational purposes and does not constitute an offer in any sense. Products with PIPELINE & EARLY DEVELOPMENT STATUS under patent (SPC) protection in Poland and/or other countries are not offered until expiration of the corresponding IP... -
Product Isavuconazole sulfate
DISCLAIMER
The above product is presented solely for informational purposes and does not constitute an offer in any sense. Products with PIPELINE & EARLY DEVELOPMENT STATUS under patent (SPC) protection in Poland and/or other countries are not offered until expiration of the corresponding IP... -
Product Enzalutamide
We provide with Anhydrous form R1
Enzalutamide is a second-generation androgen receptor inhibitor used to treat castration-resistant prostate cancer and metastatic castration-sensitive prostate cancer. Enzalutamide is an androgen receptor (AR) inhibitor for the treatment of c... -
Product Isavuconazole
under development -
Product Sacubitril Valsartan
Crystalline polymorphic form
Sacubitril/valsartan is the first agent to be approved in a new class of drugs called angiotensin receptor neprilysin inhibitor (ARNI). The medication is FDA-approved to treat patients with chronic heart failure with reduced ejection fraction (HFrEF) with NYHA class I... -
Product Ticagrelor, Form II
DISCLAIMER Each product is protected by a patent in force in Poland and is therefore developed and manufactured solely for the purpose of Regulatory Submissions or R&D purposes. This product is not offered for sale in countries where the patent is valid and the offer or sale or any relat... -
Product Acetazolamide
Therapeutic Area: Cardiovascular systemEU DMF availableUS DMF no. 10383 availableCEP available Available also in injectable form Indication: Acetazolamide decreases the pressure in the eye and is used for adjunctive treatment of glaucoma, a condition in which increased pressure in the eye can lead to ... -
Product Aripiprazole
Therapeutic Area: Nervous SystemEU DMF availableUS DMF no. 23314 availableCEP available Indication: used to treat the symptoms of psychotic conditions such as schizophrenia and bipolar disorder (manic depression). It is also used together with other medications to treat major depressive disorder in adult... -
Product Carbamazepine
Therapeutic Area: Nervous System EU DMF availableUS DMF no. 26266 availableCEP available Indication: Carbamazepine is an anticonvulsant and mood-stabilizing drug used primarily in the treatment of epilepsy and bipolar disorder, as well as trigeminal neuralgia. -
Product Carvedilol
Therapeutic Area: Cardiovascular systemEU DMF availableUS DMF no. 17060 availableCEP available Indication: Carvedilol is a carbazole derivative acts as anti-hypertensive agent. It is formulated as tablets for oral route of administration. Carvedilol is indicated for the management of essential hypertens... -
Product Carvedilol phosphate hemihydrate
Therapeutic area: Cardiovascular systemEU DMF availableUS DMF no. 20633 available -
Product Clopamide
Therapeutic Area: Cardiovascular systemEU DMF available Indication: Clopamide is indicated for the treatment of oedema, hypertension. It is formulated as tablet for oral administration. It is categorised as a thiazide like drug and works in similar way as the thiazide diuretics. It acts at the proximal c...
Polpharma S.A. resources (10)
-
News CPHI Barcelona 2023: Tackling the Pharma Talent Precipice – Part 2
This year at CPHI Barcelona (24–26 October, 2023) we sat down with C-suite executives and HR professionals to discuss the looming talent crisis in the pharmaceutical industry. With hybrid working persisting post-pandemic and a growing skills gap, how can the pharmaceutical supply chain adjust to a changing labour force? -
Brochure API Product List 2024
We manufacture our products by following our customers' and health authorities' most stringent requirements. DMF documentation for all our products is prepared in accordance with the latest requirements of EDQM (CEP), ICH (EuDMF, CTD), and FDA (US DMF). Material and product testing is performed in line with the European and United States Pharmacopeias. -
News KiloLab Laboratory: strategic milestone achieved!
KiloLab Laboratory – a development and production in a kilogram scale – has been launched, according to Polpharma API development strategy adopted in 2021. -
Video POLPHARMA API: TECHNOLOGY EXPANSION & STRATEGIC DIRECTION
Recent investments in Polpharma API’ capabilities will support a strategic move into complex API development and manufacturing for CDMO partners and generics companies worldwide, empowering the global supply chain.Visit us and learn how Polpharma API enters into a strategic transformation process that aims to invest in new capabilities and technologies like high containment or cryogenic to be fully implemented by 2026. However, our production capacity will increase significantly already from mid-2023!
Would like to meet our representatives and learn more, please contact us today: [email protected]; [email protected] -
News Polpharma’s strategic investment in HPAPI facility
Polpharma’s strategic investment in HPAPI facility, the first of this kind in Poland and one of the most advanced in Central Easter Europe.
-
Video How can we grow business value together?
Check out how we can create business value together in the whole pharmaceutical value chain, including API, B2B, commercial partnerships and other forms of collaboration. In the video: Markus Sieger, CEO of Polpharma Group, David Gonzalez, Commercial Director of API Business Unit, Mieczyslaw Starkowicz, Head of B2B Venture and Simon Clark, Commercial Director for Polpharma Group and strategic partners. -
Brochure Polpharma & Farmaprojects FDF Dossier List
We offer an attractive portfolio of FDF focused on the newest and most prescribed therapies, having a customer centric approach through a multidisciplinary support team: Sales, Marketing, R&D, RA, QA&QC, IP, etc. Due to combined efforts of Polpharma and its strategic partner Farmaprojects we guarantee supply continuity in EU and non-EU territories. -
Video Strategic investment HP API facility
In 2022, we are opening a new chapter in the history of Polpharma in the field of active substances. With the strategic investment in a HPAPI facility, Polpharma and Poland are becoming a strong pillar of the European pharmaceutical industry in the coming decades.
Grand opening of new R&D & Production Facility in Q3 2024
Completion of this investment is scheduled for the first quarter of 2024. It will include separate process laboratories (PDL), analytical laboratories (ADL) with the possibility of performing Quality Control analyses, and a GMP kilo-lab production line with volume up to 1.5 kg with potential volume increase.
Use link to read more: https://www.api.polpharma.com/news/polpharmas-strategic-investment-in-hpapi-facility-the-first-of-this-kind-in-poland/
-
Brochure Polpharma API CDMO offer
Polpharma API CDMO (Contract Development and Manufacturing Organization) supports emerging and large pharmaceutical customers in the development and commercialization of their small-molecule APIs clinical candidates thanks to +70 years of experience in process development, scale-up, and cGMP manufacturing of small molecule APIs in volumes ranging from kilos to tens of tons.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

























.png)
.jpg)








.png)



