A Day in the Life of a Global CDMO Chapter Lead – Manufacturing
.png)
The 'Day in the Life of' series has covered many aspects of the pharmaceutical pipeline, including R&D and procurement, now we're taking a look at manufacturing from a global CDMO perspective.
In this interview, Shilpi Ghosh, Global CDMO (External Manufacturing) Chapter Lead at Roche gives us an insight into her daily life. Ghosh has been a knowledgeable and valued speaker with CPHI and the opportunity to get to know her better has been a pleasure.
Please could you outline your background and the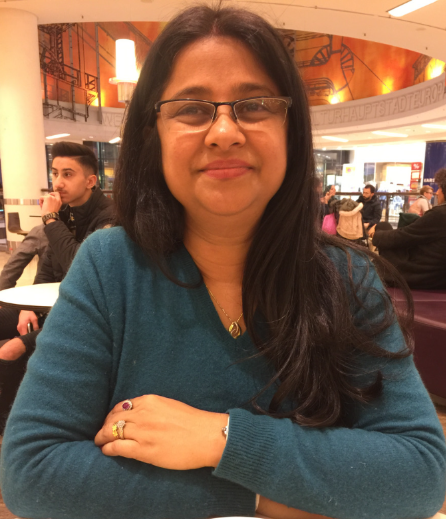 career path that has led you to your current position?
career path that has led you to your current position?
I am a Chemical Engineer from India. I started in core manufacturing in India. My experience during my career has been around Manufacturing, ERP implementation, Product/Network Strategy, and Divestment/Contract Management. With this wide experience, I am currently in a senior leadership position within the External Manufacturing and CDMO space.
What would be the perfect start to your day?
The perfect start to my day would be with a Surya Namshkar (Yoga) and a cup of good coffee while scrolling through the news. This would be followed by a scan of the days schedule that gives the feeling of an upcoming productive day.
Could you describe a typical day in your role?
A typical day can be a mix of different things. While checking in with people and team huddles or meetings remain to be a significant part of the day, other activities like operational escalation management, cross CDMO project sponsorship, and CDMO management meetings are part of the daily schedule. I do sometimes block out a part of the day for reflection and doing some self-work.
What do you most like about your role?
The most exciting part of the role is motivating my team to drive excellent results that directly contribute into the organisation objectives and making medicines available for the patients.
What made you consider getting into your field in the first place?
I always loved science and logic. Having completed my chemical engineering studies, I decided to go into manufacturing and continued the journey thereafter.
What are the biggest challenges you face? What issues affect your role?
The biggest challenges are unforeseen events that impact supply to patients. Under the circumstances keeping calm and ensuring quick resolution can sometimes be a big challenge. These unforeseen incidents also have a big impact on planned activities for myself and the team. Therefore being agile is a great asset.
What would you consider your biggest achievement to date, what are you most proud of?
It goes a long way back. While I was still in manufacturing in India, implementing ERP, I had operators who could not start a computer. I had to literally put my hand on their hand and teach them how to browse. When I completed the ERP implementation the same operators were opening requisitions and orders in SAP and entering production/maintenance data. This was a strong sense of achievement in being able to take the team to a different level.
What advice would you give to other people aspiring to your position or getting into this field?
If you are passionate about operations and are not afraid to get your hands dirty then do follow a career in manufacturing/external manufacturing. The sense of accomplishment to be able to serve patients is very high.
What do you see as the next big opportunity in your sector?
The next big opportunity in the pharmaceutical sector is to serve mass diseases with effective medication at affordable cost across the globe. This becomes even more important with a growing population till the end of this century.
How do you incorporate wellbeing into your day?
I do yoga, walk, and cook good food.
Is living sustainably important to you and how do you incorporate being environmentally friendly into your day?
Yes, it is extremely important to me to leave behind a sustainable planet. I use water and electricity mindfully, and of course minimise use of or reuse plastics.
If you weren’t in this field, what would you being doing?
As an alternative I loved English language. So another choice of career would have been Journalism.
When you were younger, want did you want to be when you grew up?
My mom. She is a mathematician and was a scientist.
Related News
-
News How to disrupt an industry as big as pharma for the better?
In this interview, hear from Matthew Wise, Head of Data at CCD Partners, on the companies they've been looking into that are offering new and interesting perspectives that have the potential to shake up the pharmaceutical industry, and how they'... -
News CPHI Pharma Awards 2024: Meet the winners from the CPHI Celebration
This year we had a lot to celebrate, the 35th Anniversary of CPHI, and our esteemed award winners, of which we included two additional categories this year, the Future Leader award, and Woman of the Year award. -
News CPHI Milan 2024 - From the Floor
Milan and CPHI welcome you to 2024 CPHI Milan! As we celebrate the 35th edition of our flagship CPHI show, editors Vivian Xie and Lucy Chard bring you the latest from the show floor, conference sessions, and innovative solutions from all exhibitors, at... -
News AstraZeneca invests in AI collaboration for cancer drug trials
The British-Swedish pharmaceutical giant is partnering with biotechnology firm Immunai Inc to increase the efficiency of some cancer drug trials. -
News CPHI Podcast Series: The power of proteins in antibody drug development
In the latest episode of the CPHI Podcast Series, Lucy Chard is joined by Thomas Cornell from Abzena to discuss protein engineering for drug design and development. -
News Eisai Alzheimer’s drug authorised in UK but still faces obstacles
In partnership with BioArctic AB, pharmaceutical company Eisai has been granted Marketing Authorisation by the Medicines and Healthcare products Regulatory Agency (MHRA) for its Alzheimer’s disease drug product Leqembi. -
News Sanofi invests billions into Frankfurt insulin production site
French pharmaceutical company Sanofi have announced an investment of EUR1.3 billion at their existing BioCampus site in Frankfurt am Main for the expansion of insulin production. -
News Alzheimer’s drug Leqembi rejected by EU drug regulator
The European Medicine’s Agency (EMA), the drug regulator for the EU, rejected Eisai and Biogen’s Alzheimer’s drug product Leqembi, citing the drug’s slowing of cognitive decline did not outweigh the risk of serious brain swellin...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



.png)

.png)
.png)
.png)