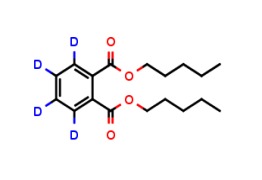
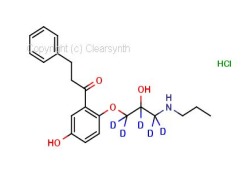
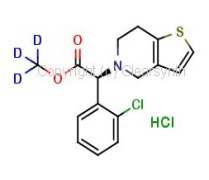
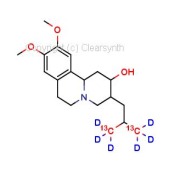
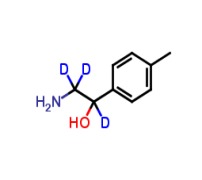
4-Acetamidobenzenesulfonic Acid-d4 Pyridine (Major)
Product Description
CLEARSYNTH LABS LIMITED

-
IN
-
2015On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
CLEARSYNTH LABS LIMITED

-
IN
-
2015On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from CLEARSYNTH LABS LIMITED (99)
-
Product Nitrosamine and Drug Impurities | Testing and Analytical Services
Clearsynth is a globally trusted partner for reference standards and research chemicals. We offer over 3,000 nitrosamine impurities, 5,000+ stable isotopes, and 10,000+ drug impurities, encompassing a vast portfolio of more than 325,000 products serving various industries, including pharmaceuticals, clinic... -
Product Reference Standards and NMR Solvents
Clearsynth provides a wide range of high-purity NMR solvents designed to ensure optimal resolution and reliable analysis, supporting both research and quality control applications
Benzene D6 , Chloroform-D , Dimethyl Sulphoxide D6 (DMSO D6) , Methanol D4 , Deuterium Oxide , Tetrahydrofuran... -
Product 2-Amino-1-(4-methylphenyl)ethanol-d3
A α-substituted phenylethanolamines that binds reversibly to the octopaminergic receptor. -
Product N-Acetyl Metoclopramide-d3
Labelled N-Acetyl Metoclopramide. N-Acetyl Metoclopramide is a related compound of Metoclopramide. -
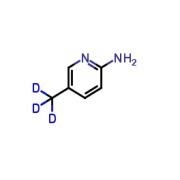
Product 2-Amino-5-(methyl-d3)-pyridine
Molecular formula: C₆H₅D₃N₂Molecular weight: 111.16 -
Product rac-cis-Ambroxol-d5
Labelled anaologue of cis-Ambroxol, the cis-isomeric impurity of Ambroxol. cis-Ambroxol is a metabolite Bromhexine. -
Product 1,4-Bis[3,4-dihydro-2(1H)-quinolinon-7-oxy]butane-d8
Labelled intermediate used in the process for the preparation of Aripiprazole. -
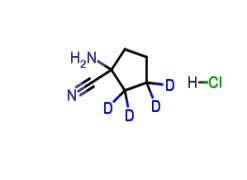
Product 1-Amino-1-cyanocyclopentane-d4 Hydrochloride
A labelled intermediate of Irbesartan, an antihypertensive active pharmaceutical ingredient. -
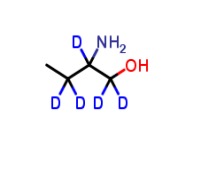
Product L-2-Aminobutanol-d5
Molecular formula: C₄H₆D₅NOMolecular weight: 94.17 -
Product Adapalene-d6 Methyl Ester
Labelled Adapalene intermediate, an ester of labelled Adapalene. -
Product Amisulpride-d5 N-Oxide
Labelled Amisulpride N-Oxide. Amisulpride N-Oxide is an impurity of Amisulpride. Amisulpride N-Oxide was identified as a photodegradation product of Amisulpride. -
Product (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide-d3
Labelled (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide. (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide is a related compound of Levetiracetam.
CLEARSYNTH LABS LIMITED resources (2)
-
Brochure Corporate Profile
Your Globally ''Trusted'' Partner for Reference Standards and Research Chemicals. -
Brochure Deuterated Products
Clearsynth is pleased to be a global leader in deuterated chemistry. We manufacture from 'mg' to 'tonnes' in our state-of-the-art manufacturing facility.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance












![1,4-Bis[3,4-dihydro-2(1H)-quinolinon-7-oxy]butane-d8](https://www.cphi-online.com/46/product/107/87/83/p0th_S.jpg)

















.jpg)