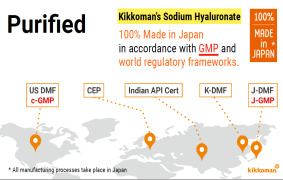
100% made in Japan & meet GMP and world wide regulatory frameworks
Product Description
Kikkoman Biochemifa Company

-
JP
-
2017On CPHI since
-
4Certificates
-
100 - 249Employees
Company types
Categories
Kikkoman Biochemifa Company

-
JP
-
2017On CPHI since
-
4Certificates
-
100 - 249Employees
Company types
More Products from Kikkoman Biochemifa Company (6)
-
Product Sterile Grade at ISO Class 5 facility
Kikkoman can offer "sterile grade” products, purified through a super purification process at our ISO Class 5 facility, approved by the Japanese Authority. -
Product Customized Intrinsic Viscosity (I.V.) -Effectiveness and safety
Kikkoman can offer customized intrinsic viscosity ranges, which are refined through our special process controls, ranging, in 6 grades, from low to high Intrinsic Viscosity (I.V.). This I.V. control is necessary to ensure effectiveness and safety of the finished product. -
Product Halal Certification
Halal certification by the Japan Muslim Association is available. It facilitates our promotion to Middle Eastern, North African and South-East Asian markets. -
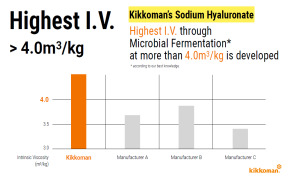
Product Highest Intrinsic Viscosity through Microbial Fermentation
Kikkoman is developing I.V. through Microbial Fermentation at more than 4.0 m3/kg, which is, to our knowledge, the highest Intrinsic Viscosity through Microbial Fermentation. -
Product Kikkoman Sodium Hyaluronate meets your needs
Kikkoman Sodium Hyaluronate with variety of I.V. from low to high meets with our client’s various needs such as replacement from the one currently using, adding as 2nd or 3rd source and using new development of finished product. -
Product Pharmaceutical Grade Sodium Hyaluronate Line up
We have 7 grades in total differentiated by intrinsic viscosity and endotoxin which are important for the effectiveness and safety of the finished product. All specifications, including Intrinsic Viscosity, can be customized by GQP contract.
The applicable endotoxin level can be 0.05 or 0...
Kikkoman Biochemifa Company resources (4)
-
Video Welcome message and Introduction of Sodium Hyaluronate/Hyaluronic Acid
Welcome message and Introduction of Sodium Hyaluronate/Hyaluronic Acid. 1. What makes your product different from others ? 2. What impact of viscosity control to the customers? 3. Relation between viscosity and application 4. Sterile Grade of sodium hyaluronate -
Video Kikkoman: “100 years in 100 seconds”
The dream born 100 years ago and the progress taken since then have been arranged into a short movie. You can watch it here! -
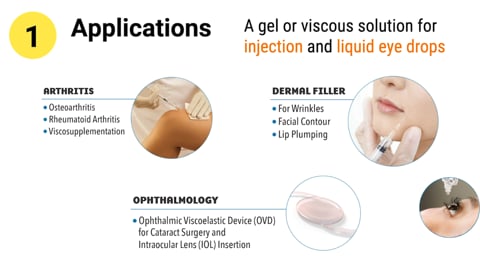
Video Applications and Grade
1. Gel or viscos solution for injection and liquid used by arthritis, dermal filler, eye surgery, coating agent and tissue engineering etc.2. Seven grades in total.
3. Regulatory Cert. GMP, DMF, CEP etc. -
Video Four features of Sodium Hyaluronate/Hyaluronic Acid
Four features of Kikkoman Sodium Hyaluronate for Pharmaceutical and Medical device1. Purified to be met with GMP and international standard
2. Sterile Grade
3 Customized viscosity range
4. More than I.V. 4.0m3/kg
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance