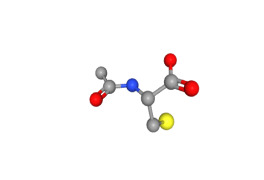
Guaifenesin_USP/CEP/EP/JP/BP

Product Description
Seven Star Pharmaceutical

-
TW
-
2023On CPHI since
-
4Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
Seven Star Pharmaceutical

-
TW
-
2023On CPHI since
-
4Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from Seven Star Pharmaceutical (2)
-
Product Acetylcysteine_ USP/CEP/EP/ChP
Indication:1. treatment of respiratory affections characterized by thick and viscous hypersecretions due to acute bronchitis, chronic bronchitis and its exacerbation, COPD, pulmonary emphysema, mucoviscidosis and bronchiectasis.
2. Antidote in acetaminophen poisoning.
-
Product Gadobutrol Monohydrate
MRI Enhancing Agent
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance