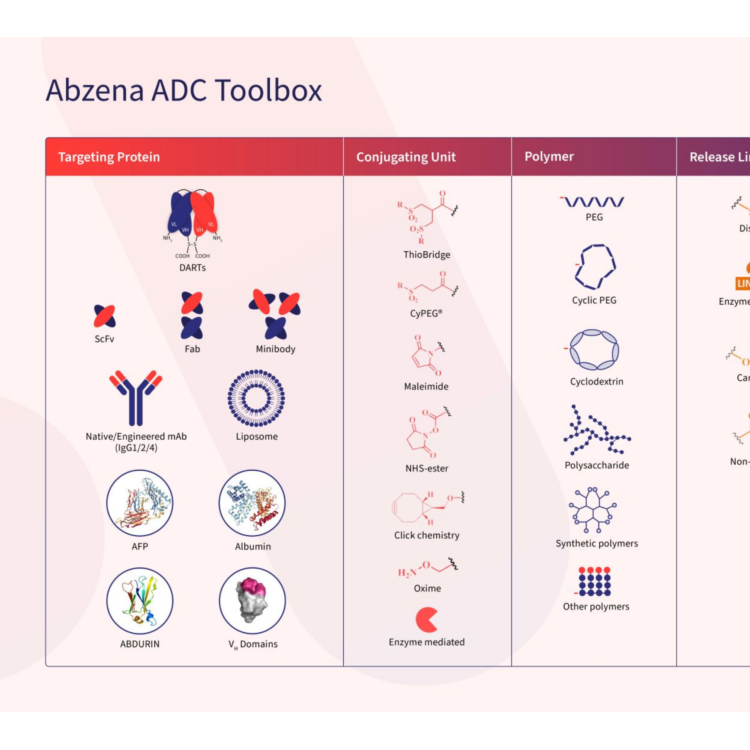
Biologics process development and analytical method development

Product Description
Abzena

-
US
-
2018On CPHI since
-
250 - 499Employees
Company types
Primary activities
Categories
Abzena

-
US
-
2018On CPHI since
-
250 - 499Employees
Company types
Primary activities
More Products from Abzena (2)
-
Product Bioconjugation & Complex Chemistry Development and Manufacturing
Specialized ADC, bioconjugate and linker payload services. Moving medicines created with ADCs, bioconjugates and linker payloads forward is where we’re at our best. And it’s made Abzena a leader in delivering on their promise. Building on the unmatched expertise of our scientists, the IP we’ve generated,... -
Product Lead Discovery and design
Abzena’s antibody discovery team collaborates with you to build the foundations of a successful program and de-risk your development path. We deliver expert assessments of target molecules, immunization strategies and antibody screening cascades that inform your selection of the best candidates for develo...
Abzena resources (2)
-
News Abzena and BioXpress Therapeutics partner to provide global biosimilar support
The combined capabilities will offer flexibility and innovation from small-scale biosimilar development through to large-scale manufacturing -
News Abzena to build US biologics manufacturing plant in North Carolina
New facility will be research partnering company’s sixth global site and will be dedicated to mammalian biologics
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance